 |
||||||||||
Date: March 6, 2025
by Chaya Venkat
Related Article: IDEC 152

Anytime there is a new monoclonal antibody that looks like it may have a real impact on CLL, you can bet it gets my attention. I am happy to report, there is a new babe in town, and her name is “Lumi”. Actually, to be entirely honest, the name is “Lumiliximab”. But if you are like me, I think you will like the handle “Lumi” better — it is a whole lot prettier and it is easier to spell. So, until the company (Biogen Idec) comes up with a better name, I am going to stick with “Lumi”. I had a friend in grad school with that name, and all the guys thought she was quite the sexy ‘babe’.
Let’s see: so far we have Rituxan, Campath and, hopefully, Humax-CD20 in the near future (with any luck, FDA approval for HuMax-CD20 will come in 2025). Now Lumi is making her debut, in a major Phase III clinical trial designed to get FDA approval for use in CLL. A total of 276 patients will be recruited for this trial, at many locations across the US, Canada, Australia and Europe. If your CLL puts you into the therapy market in the near future, you might want to read up on this new drug and its pivotal clinical trial, as a possible path forward. In this article we plan to give you the scoop on Lumi, what it targets, how it is thought to work, its possible risks and rewards and details of the clinical trial. The company seems to have adopted an aggressive strategy for getting this drug approved. If all goes according to plan and the FDA obliges, Lumi may be commercially available to CLL patients in late 2025.
Monoclonal antibodies such as Rituxan and Campath are considered smart bombs in that they home in and attach themselves to specific markers on the tumor cells. In the case of Rituxan and HuMax-CD20, that marker is CD20. This marker is present only on mature B-cells (and not on stem cells or other cell lines), making both of these drugs very specific in their targeting. Unfortunately for us, CD20 is only dimly expressed in CLL (there are only a few thousand CD20 markers per CLL cell, compared to many more thousands of CD20 markers per cell in non-Hodgkin’s lymphoma patients) and we have discussed before how this impacts Rituxan’s efficacy as a single agent in CLL and how it may be less of a stumbling block in HuMax-CD20 therapy (A Smarter Monoclonal on Trial). Campath targets CD52, a marker present on other cell lines besides B-cells, a fact that makes Campath not quite as specific as Rituxan or HuMax-CD20, resulting in more "collateral damage" from Campath therapy.
The marker that is targeted by Lumi is CD23. Those of you who have done your homework and learned about the wide variation in the very specific signatures of CLL cells (What Type of CLL Do You Have?) know that expression of CD23 is a defining characteristic and a required feature of all CLL cells. In fact, the lack of CD23 expression raises serious doubts about a CLL diagnosis in the first place. Putting it another way, here is one situation where we no longer have to worry whether or not your particular brand of CLL expresses the marker of interest. All CLL cells express CD23.
Looking a bit closer at the picture, researchers have found that the CD23 construct is attached to the cell surface by a kind of stalk. Sometimes the stalk is torn off, in which case the CD23 is broken away from the cell and floats free in the blood. People have measured the amount of this type of free “soluble” CD23 (sCD23) floating around. There has been quite a bit of work suggesting that higher levels of sCD23 is actually a prognostic indicator for rapid proliferation and aggressive disease. In fact, it has been suggested that cell-bound CD23 plays an important role in activating CLL cells and kick-starting the process of proliferation of the cancer cells. I have more to say about this, in the Editorial section below. As you can read in the following abstract (as well as the full text of the article, available free of charge by clicking on the link provided), CD23 plays an important role in the progression of CLL and for that reason it is a logical target in this disease.
Link: Free full-text article in Blood.
Blood. 1994 Sep 15;84(6):1881-6.
Role for low-affinity receptor for IgE (CD23) in normal and leukemic B-cell proliferation.
Fournier S, Rubio M, Delespesse G, Sarfati M.
Allergy Research Laboratory, Notre-Dame Hospital Research Center, University of Montreal, Canada.
CD23 gene is overexpressed and abnormally regulated in the most frequent adult leukemic disorder, B chronic lymphocytic leukemia (B-CLL). Switch on and off in the upregulation of surface CD23 expression consistently occurs in the early stage of normal B-cell activation, suggesting a key role for CD23 in this process. We show here that, after ligation of mlg in the presence of interleukin-4, the increase of CD23 protein precedes B-cell DNA synthesis and mainly results from the strong induction of CD23 type-B isoform. Exposure of normal B cells to conventional or phosphorothioate-derivatized CD23 antisense oligonucleotides (predominantly type B) significantly augments B-cell proliferation induced by antigen receptor stimulation or direct contact with activated T cells. Unexpectedly, CD23 antisense, but not sense, oligonucleotides specifically enhance rather than suppress CD23 expression on B cells. Finally, a selective increase in CD23 type-B expression provokes the entry of resting (Go) CLL B cells into G1 and S phase of the cell cycle in the absence of any other stimulus, whereas it synergizes with tumor necrosis factor-alpha to increase the number of activated B cells. These results provide compelling evidence that CD23 represents an important molecule directly involved in the process of normal or leukemic B-cell activation and growth.
PMID: 8080994
____________
The fact that the stalk holding the CD23 connected to the CLL cells is prone to snap off and “shed” the CD23 construct into the blood plasma does complicate things a bit. It means a sufficient amount of Lumi has to be given to completely saturate all of the soluble CD23 floating around — and then some more of the drug to get to work on the cell bound CD23. You will notice that the dosage for Lumi is 500 mg/m2, a tad more than the so-called standard of 375 mg/m2 for Rituxan. Pre-clinical work with Lumi as well as the early Phase I study of Lumi as single agent in relapsed CLL patients indicated that at this dose there is enough of the Lumi around to saturate the free sCD23 as well as attack the CLL cell-bound CD23 marker.
A more important question that occurred to me is whether the CLL cell can shake off the CD23 after it has been tagged by Lumi. As a child I used to watch geckos hunting each other on the walls of our home in India. When a large gecko grabs on to the tail of a smaller one, the victim often escapes capture by shedding off its tail and running for cover. The sacrifice of its tail has the huge benefit of saving its life and a new tail grows back soon enough. I wondered if CLL cells can do a similar trick, shed off their CD23 along with the Lumi attached to it, in an attempt to survive the onslaught. I asked this question in our long 2-hour telephone conference call with Biogen Idec. I am not sure anyone knows the definitive answer but it seems reasonable to me that some amount of target shedding does happen, with the CD23-Lumi combination getting stripped off, not unlike the CD20 “shaving reaction” we discussed earlier (CD20 Shaving with Rituxan).
Since CD23 is expressed by other cell lines besides mature B-cells, the question arises whether Lumi therapy targets these cell lines as well. You may remember from reading the many Campath articles we have on our website (see our directory page on Campath Therapy), targeting multiple cell lines contributes to unwanted side effects. In the case of Campath, since CD52 is expressed on T-cells as well as B-cells, Campath therapy invariably leads to massive T-cell depletion, and the resulting risk of viral reactivation (CMV, hepatitis, shingles, TB, pneumonia, etc.) is a huge issue for this drug. In the case of Lumi, my trusted “bible” (“Immunobiology” by Charles Janeway) confirms CD23 is expressed by platelets, dendritic cells and activated macrophages. Platelets under attack? That got my attention. CLL patients are not exactly rich in platelets and many of us have preexisting issues with decreasing platelet counts either as a result of packed bone marrow not making enough of them, autoimmune disease killing them off or a malfunctioning and clogged up spleen sequestering perfectly good platelets and taking them out of circulation.
During our conversation with the company we spent quite a bit of time on the subject of potential platelet destruction as an unwanted effect of Lumi therapy. I am happy to report this is not a major concern. It appears the level of CD23 expression on B-cells is far higher than the level expressed by platelets. Using the “proof of the pudding is in the eating” principle, I was relieved to note that Lumi has been in human trials for asthma and in these non-cancer patients it showed no propensity for unwanted platelet destruction.
Now the matter of toxicity to T-cells. Below is a link to the full length article describing the allergy trial using Lumi, in case you are interested. This was a phase I trial intended to evaluate the safety of Lumi administered as a single dose. (Don’t get confused by the name. Back then Lumi used to be called IDEC-152. I still think the name Lumi is prettier than IDEC-152 or lumiliximab!) No significant toxicity was reported in the 24 patients who received single doses of Lumi, even at the maximum dose of 15.0 mg/kg. The researchers demonstrated that there was no T-cell depletion, and therefore as one would expect, the infection rates were similar between patients treated with either Lumi or placebo.
I was also happy to note that patients did not have abnormal platelet counts or bleeding times in this trial. Mouse work is all good and fine, but any day of the week I like to see a low toxicity profile demonstrated in real live human beings. This earlier allergy work with Lumi demonstrating very low human toxicity profile has set my mind at ease on the important issues of platelet and T-cell destruction.
Here is a link to a pdf version of the paper on this phase I allergic asthma clinical trial: IDEC 152 in Allergic Asthma.
Those of you who like to read the detailed story and not just the cheat-sheets know by now that monoclonal antibodies have a number of mechanisms by which they can kill the cells they target. I recap them very briefly below (but here is a link where you can read more about these matters at your leisure: Sons of Rituxan and Campath).
Direct cell kill. This is the process in which the monoclonal antibody attaches to the hapless cancer cell it is targeting and kills it dead without needing help from any other source. Radiolabeled monoclonals such as Bexxar and Zevalin carry a toxic payload (radioactive material) that kills the cell by means of the radioactivity. Others (such as Ontak) use a chemical toxin rather than radioactivity to kill the target cell. Even “cold” monoclonals that carry no additional payload (such as Rituxan, HuMax-CD20 and Campath) can do a certain amount of direct cell kill, forcing the cell to listen to the command to commit honorable suicide (apoptosis). Here again, there is a distinction between direct cell kill that requires the p53 pathway (often by blowing up mitochondria, the power-houses present in each cell) and direct cell kill that works independent of the p53 pathway. Unfortunately, there seems to be little doubt that the direct cell kill method used by Lumi requires an intact and functioning p53 pathway. Why unfortunate? Because many fludarabine-refractory CLL patients have deletions or mutations in this important tumor suppressor pathway, because of deletions or mutations at the 17p (p53) gene locus. CLL cells without a functioning p53 pathway will turn a deaf ear to Lumi’s request for honorable suicide. The company feels it is probably too early to say a lot about efficacy against 17p-deleted CLL given that only 4 patients in the trial had this defect. However, the pointers are fairly clear to me, given the results and Lumi's principal mode of action. I am pleased that the company plans to follow up on its preliminary data to shed more light on the 17p issue. If there is new information on this front, we will certainly bring it to your attention.
Complement mediated cell kill. Complement is a very potent and important mechanism used by our bodies to defend us against dangerous pathogens. It is an ancient mechanism and one we share with most of the animals living in our world. It evolved a long time ago, well before multi-cellular organisms like you and me came on the scene, before the advent of newfangled stuff like T-cells. Complement does not need a whole lot of coaxing to get to work: when it sees a cell (or pathogen) tagged by an appropriate antibody (such is the case when a CLL cell is tagged by drugs such as Rituxan, Campath or Humax-CD20), it goes to action. One of the ways in which it kills is called the “MAC Attack”, where complement literally punches holes in the outer skin of the cell or pathogen and the hapless victim leaks out its contents and “bleeds” to death. To protect against too much of a good thing, our bodies have established a threshold below which complement will not attack. And as we discussed before (A Smarter Monoclonal on Trial), this may explain why Rituxan does not do as good a job of harnessing the power of complement dependent cytotoxicity (CDC) as HuMax-CD20 does.
Based on the pre-clinical work (soon to be published) on Lumi, it does not appear that Lumi triggers direct cell kill via complement. The good news is that since complement activation is not a major pathway for Lumi, there are far fewer infusion related side effects, the pesky “shake and bake” phenomenon associated with other monoclonals, not much of the hives, rashes, low blood pressure, etc. In fact, there are so few infusion-related side effects with Lumi that it is given in a pretty concentrated form, just 150 ml. of saline in the bag and not the extra large size infusion bags we have come to expect. I am told the actual Lumi infusion can be done in as little as 2 hours, a lot faster than the pretty much whole day affairs of HuMax-CD20 and Rituxan infusions.
Cell kill with a little bit of help from friends. Cellular cytotoxicity (ADCC or CDCC) requires the cooperation of other parts of the immune system. Cell lines such as T-cells, NK cells, neutrophils and macrophages take part in killing targeted cells. This is possibly the most important mechanism for Rituxan, whereby tagged CLL cells are killed by T-cells, neutrophils, etc. It may also explain why Rituxan does not work very well as a single agent in late stage or refractory / previously treated CLL patients. Chances are that late stage and refractory patients have inadequate immune function, with compromised T-cells and reduced counts of neutrophils. Without adequate help from these other cell lines, ADCC and CDCC cannot work very well. This mode of action is likely to be weak at best with Lumi.
Synergy with chemo. All of the monoclonal antibodies (Campath, Rituxan, HuMax-CD20 and Lumi) seem to synergize with standard chemotherapy drugs. The word “synergize” means 2 plus 2 equals more than 4: the combination of the monoclonal antibody plus chemo works better than just the sum of the effects of the two drugs. That is good news indeed. It means we may be able to get more of a bang for the buck, by adding monoclonal antibodies to more conventional chemotherapy regimens. I just wish researchers took the opportunity presented to reduce the dosages of the chemo components while increasing the role of the monoclonals, rather than going for “manly” doses of maximum levels of each drug — and more the merrier. One of the reasons why we have strongly endorsed the FCR Lite trial underway at University of Pittsburgh Medical Center is that it uses this concept of controlling toxicity. Results from the UPMC trial have been compelling. Dr. Foon has reported terrific responses and lot less toxicity, compared to the conventional FCR regimen.
Bottom line, Lumi seems to work via direct suicide signals to the target cell and the apoptosis signaling does require a functional p53 pathway. It does not activate complement to any large extent and therefore does not use complement mediated pathways, with or without the help of other cell lines. That limits its fire power, but at the same time it also means a lot less infusion-related adverse effects. My guess is that Lumi infusions will be quick and relatively painless, easily tolerated by most patients. With this cell kill profile, it is understandable that the early Phase I trial of Lumi as single agent in previously treated patients (with p53 defects likely) showed no complete or even partial responses. It seems that Lumi will be most effective in patients with intact p53 and most likely in combination with other drugs.
The graphs below come from a slide presentation at the 2025 ASH Annual Meeting by Byrd, et al. These charts reflect pre-clinical work with mice. As you can see, adding Rituxan or fludarabine makes Lumi work whole lot better.
Disease Free Survival of SCID Mice:
Lumiliximab/Fludarabine and Lumiliximab/Rituxan
Slide Presentation at ASH 2025, Byrd, et al.
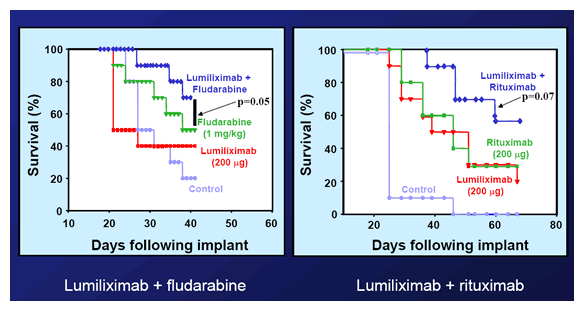
One of the highlights of the recent ASH 2025 conference PC and I attended was the presentation of the results of a Phase II study using a combination of FCR + Lumi. This was an open label, single-arm study conducted at multiple locations, for late stage or relapsed CLL patients. Dr. Byrd's elegant PowerPoint presentation had a wealth of information for all patients considering Lumi therapy. It described much of the information we have discussed above, as well as the full details of the Phase II results.
I have chosen to use the content from a couple of the slides in their presentation, since they highlight the response comparison between FCR + Lumi versus plain FCR. Bear in mind, this is a bit of an apples-to-oranges comparison, since the comparison is between this single arm FCR + Lumi study and the earlier FCR study done at M. D. Anderson. While this admittedly rough-and-ready comparison showed no increases in the percentage of overall response, there were a significantly higher number of complete responses. Only a well conducted, double-arm study where well matched (randomized) patient cohorts are inducted into the FCR and FCR + Lumi arms will be able to confirm whether the higher percent of CRs for the combo with Lumi is something we can take to the bank. The good news is that the addition of Lumi to FCR did not increase the toxicity levels. The patients in the Phase II FCR + Lumi trial had “only” the usual toxicity we have come to expect from FCR. (I have to admit to a degree of bitterness when researchers talk about “maximum tolerated dose”, “non-overlapping toxicity” or “acceptable toxicity” with a certain amount of nonchalance. We who are on the sharp end of the infusion needle see things just a bit differently). Among the results from this study (soon to be published in Clinical Cancer Research) Dr. Byrd showed that lumiliximab had no affect on T-cell and NK cell numbers and atypical infections were not observed. This would be an indication that Lumi does not produce immunosuppression.
| Regimen | FCR + Lumi | FCR |
| Number of Patients | 31 | 177 |
| Overall Response | 72% | 73% |
| Complete Response | 52% | 25% |
As I expected, Lumi did not do much for the 17p (p53 deleted) patients, even with the backup of FCR. There were four of them. Only one of them got any response worth writing home about and that, too, was a partial response.
| FISH Abnormality | Number of Patients | Overall Response | CR/PR |
| 11q (ATM) deletion | 8 | 6 | 5/1 |
| 17p (p53) deletion | 4 | 1 | 0/1 |
Biogen Idec have put their money where their mouth is, with a recently announced clinical trial that compares FCR + Lumi head-on with the present day “gold standard” FCR. (I have my pet peeves with any therapy regimen that declares itself the gold standard, without the benefit of large scale, multi-center, double arm comparison studies. But that is a different story). Below are the link to the announcement on clinicaltrials.gov and my commentary. http://clinicaltrials.gov/show/NCT00391066.
Now that you know a bit more about Lumi, I would like to show you how the new monoclonal antibody stacks up against the other monoclonals. This cheat sheet summarizes most of the details we discussed above, but please be aware that as with all cheat-sheets, some of the detail is lost when we opt for the convenience of an “easy” summary.
| Antibody | Campath | Rituxan | HuMax-CD20 | ‘Lumi’ |
| Type | Humanized | Chimeric (mouse) |
Human | Primatized (monkey) |
| Target Marker | CD52 | CD20 | CD20 | CD23 |
| Expression on Mature B Cells |
Bright | Dim | Dim | Bright |
| Other Cell Lines with this Marker |
T-cells, macrophages, neutrophils, sperm |
None | None | Macrophages, dendritic cells, platelets |
| Mechanism of Action | ||||
| Direct Apoptosis | Yes | Yes | Yes | Yes |
| Complement (CDC) | Yes | No | Yes | No |
| Effector Cells (ADCC) | Yes | Yes | Yes | No |
| Synergy with Chemotherapy |
Yes | Yes | Yes | Yes |
| Claim to Fame | Works in p53 deleted cases |
Low toxicity;
|
Low toxicity; Very specific B-cell target; may work with even dim CD20 |
Low toxicity Ineffective as single agent |
| Adverse Effects | ||||
| Infusion Related | Yes | Low | Moderate | Low |
| T-cell Depletion | Yes | Low | Low | Low |
| Viral Reactivation | Yes | Low | Low | Low |
| Neutropenia | Yes | Delayed onset | ? | ? |
| Pivotal Clinical Trials | ||||
| Single Agent | Single Agent | Single Agent | Single Agent | |
| IV & Sub-Q | IV & Sub-Q | IV Only | IV Only | |
| F + Cam | RF, FCR | FC + H | FCR + L | |
| FCR + Cam | PCR | |||
| HDMP + Cam | FCR Lite | |||
Abbreviations: F: fludarabine; P: pentostatin: C: cyclophosphamide; R: Rituxan; H: Humax-CD20; L: lumiliximab; HDMP: High dose methylprednisolone; Cam: Campath; IV: intravenous infusion; sub-Q: subcutaneous injection. |
||||
The new Phase III double-arm clinical trial announced by Biogen Idec is a bold move. The company hopes that they will get FDA approval if this trial demonstrates improved rates of complete responses for FCR + Lumi in a direct head-to-head comparison with FCR. I can understand their logic — this is what it takes to get the drug approved for use in CLL, and it is the approach that makes most business sense.
However, I wish our system allowed for approaches other than these “devil-and-the-deep-blue-sea” comparisons. It is no consolation to me that Lumi does not add to the toxicity profile of FCR. We are starting with a bleak enough list of toxicities with just the FCR regimen!! In the real world where companies need to get FDA approval to market their drug as soon as possible, there may be no choice other than to compare response stats with the biggest and baddest “gold standard” out there. I guess we need to be thankful the comparison is not between (FCR + Campath) and (FCR + Campath + Lumi).
I did raise this issue with the company and expressed my desire to see clinical trials that paid more attention to the toxicity side of the equation. For example, based on the encouraging success of FCR Lite, how about a FCR + L “Super-light”? Why not use synergy to our advantage and maintain the efficacy while reducing toxicity? For that matter, since there seems to be some synergy between Rituxan and Lumi, how about a R + Lumi combination? There is precedent for this double monoclonal approach, since we have already witnessed Rituxan + Campath combinations (Rituxan plus Short Duration Campath).
Last but not least, I wish the researchers would explore further the role of CD23 marker in active proliferation of CLL. In previous articles we have discussed the potential value of 17-AAG ( see 17AAG Clinical Trial and 17AAG), a drug that controls the functioning of ZAP70, which in turn is implicated in giving CLL cells the signal to “live long and prosper”. If (as suggested in the abstract we cited way up in this article) CD23 is part of the proliferation machinery of CLL cells, tagging CD23 markers with Lumi may not be enough to kill the cell directly — but it may be enough to gum up the works and slow down the rate at which the cell can divide and multiply. In other words, are we looking at a drug that may not work like gangbusters by killing CLL cells at first contact but instead may be the best thing available at keeping the CLL from proliferating? The low toxicity profile of Lumi demonstrated in the earlier allergy work suggests it might be an acceptable candidate for a maintenance approach in CLL.
I would like to see creative trials structured around getting patients into remission using other drugs (hopefully low toxicity combinations), followed by Lumi at regular intervals to keep the remissions going. Many chemo naïve patients get reasonable CLL clearance with single agent Rituxan. The problem is that the remissions are disappointingly short-lived and all too soon the CLL counts start increasing. Recent work by Chiorazzi, et al., at LIJH have demonstrated that the rate of proliferation in CLL cells can range all the way from 0.01% to 1% per day. (See the Chiorazzi article in the 2025 ASH Education Book: Evolving View of the In-Vivo Kinetics of CLL.) The guys with the 0.01% per day are the lucky ones who can stay indefinitely in watch and wait. The guys with the 1% or higher proliferation rate will have doubling times measured in a few months, not years. Here then is the million dollar question: can Lumi help reduce the proliferation rate and change fast-paced hares into slow moving tortoises? I don’t know about you, but I would jump at the chance to tame the proliferative drive of aggressive CLL and make it a more indolent cancer that allows patients to enjoy longer remissions — by means of a drug that does not carry the penalty of added toxicity.
Last but not least, I would like to compliment Biogen Idec on their efforts to reach out to the patient community. They made the effort to contact CLL Topics, to schedule the conference call and patiently respond to my long laundry list of questions. They made sure I got all the documents I asked for, promptly. This detailed review of Lumi would have happened sooner or later but it would have been harder without their help. Most of all, I am impressed that they were willing to listen to our concerns and to do what they could to address some of them. I told them CLL Topics would always be willing to support new clinical trials that explore low toxicity approaches. Biogen Idec is rich enough and is not going to need our money to conduct clinical trials but constructive feedback from the patient community is perhaps even more valuable.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2025-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
