 |
||||||||||
Date: December 13, 2025
by Chaya Venkat
Related Articles: HuMax-CD20

If drug companies were not competitive we would not see new drug development at rates that can make a difference to today's patients. Fortunately for us, that is not the case. The billions of dollars Rituxan makes for its owners has painted a big red "O" on this drug and many different companies are gunning for a piece of that action. Today I would like to tell you a little bit about an exciting new development that is here and now: a new version of Rituxan. It is a new construct that is ready for phase I / II clinical trials and, for a change, the protocol includes CLL patients. This clinical trial will soon to be followed by even better versions of Rituxan and Campath, the two popular anti-CD20 and anti-CD52 monoclonal antibodies, respectively. These "Sons of Rituxan" and "Sons of Campath" are no longer just wishful thinking, I am willing to bet we will see them in clinical trials in the next year or so.
Before I get into the details of this new clinical trial, let us take a little time to understand the technology. Signing up for phase I / II clinical trials is a bit like buying a new car. You need to spend a little time familiarizing yourself with the new technology, figure out if the price tag is worth it. You don't want to buy a lemon, and the best way of avoiding making life threatening mistakes is to do your homework up front. Don't tell me how you are busy being a CLL patient 24/7, there will be a pop quiz on this next week. Kidding aside, I will focus only on the "Son of Rituxan" that has announced patient recruitment.
There is still controversy about exactly how cell kill happens in a Rituxan-tagged CLL cell, but we are beginning to come up with a general picture, filling in more and more of the details. That helps in developing more potent versions of the drug. One of the best detailed and yet blissfully brief expositions I have seen to date on how Rituxan works comes from Drs. Cragg and Glennie, published on the Physician's Education Resource site. Read this and you can really wow your local oncologist at your next meeting, even without staying at a Holiday Inn Express. Here is the link: Complexity of the Antitumor Mechanisms of the Anti-CD20 Antibodies. Don't let the buzzwords throw you for a loop, they are just so much jargon that these guys in white coats use. I bet lab mice and monoclonals don't know a word of jargon and yet they get the job done. We should be able to manage as well. If, however, you prefer one of my cartoon version of how things work, read on. Some of the excruciating details may be lost, but you will get the general picture.
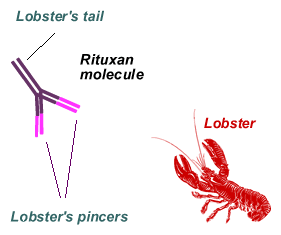 Those of you who like eating lobster should have no trouble remembering how monoclonal antibodies look, more or less. (As for me, I am a life-long vegetarian and some of my best friends are lobsters and other crusty old guys ). First, there are the two dangerous looking pincers up front. You have to imagine that the tips of these pincers are exactly correctly shaped to grab on to the CD20 markers (and only CD20 markers) exhibited by all mature B-cells. Getting grabbed by Rituxan's "pincers" destabilizes the B-cell to some extent but it is often not enough to kill it. Other systems are needed to deliver the killing blow. In addition to its pincers, our lobster has a tail as well, very important in the context of how Rituxan works. We have discussed these mechanisms piecemeal in other articles, but I thought I would put them all in one place for your convenience. Below is a cartoon of the major mechanisms. I will refer to it in the discussion below.
Those of you who like eating lobster should have no trouble remembering how monoclonal antibodies look, more or less. (As for me, I am a life-long vegetarian and some of my best friends are lobsters and other crusty old guys ). First, there are the two dangerous looking pincers up front. You have to imagine that the tips of these pincers are exactly correctly shaped to grab on to the CD20 markers (and only CD20 markers) exhibited by all mature B-cells. Getting grabbed by Rituxan's "pincers" destabilizes the B-cell to some extent but it is often not enough to kill it. Other systems are needed to deliver the killing blow. In addition to its pincers, our lobster has a tail as well, very important in the context of how Rituxan works. We have discussed these mechanisms piecemeal in other articles, but I thought I would put them all in one place for your convenience. Below is a cartoon of the major mechanisms. I will refer to it in the discussion below.
Rituxan Cell Kill Mechanisms
Landing the Punch

If you remove all the red blood cells and white blood cells, platelets, etc., from whole blood, what is left behind is called "plasma". It is a clear, amber colored liquid, that is more than 90% water. It has in it about 6% proteins, some fat globs (lipids) and salts. Some of these proteins form the "Complement" system. Unlike whole blood, plasma transfusions do not require blood type matching, since there are no live cells in it, no DNA to match.
"Complement" is a set of proteins in the plasma, that work together like a well oiled machine in fighting infections. This is what happens (should happen!) when you get a bug. Ugly antigen markers on the surface of a bacterium are tagged by roaming immunoglobulins (aka "antibodies") in your blood. This combination of antigen-antibody pair triggers a response from the complement system in the blood plasma. There are more than 20 proteins involved in the complement system. They work together to start the process of destroying and taking apart the offending bacterium, literally tearing it into pieces. Macrophages are attracted to the site of the carnage - these garbage collectors of our immune system gobble up the debris and cart it away.
If this bit of biology 101 has you cross-eyed, just remember this sound bite: in the case of a CLL patient, the CD20 marker on CLL cells is the "antigen", and it is tagged by Rituxan, our factory-made immunoglobulin. Rituxan is simply an artificially manufactured antibody targeted for a single antigen, namely the CD20 marker. Once the CLL cells have been tagged by Rituxan, forming that all important antigen-antibody pair, complement proteins rush in to start the process of cell kill.
The abstract below describes cell studies, animal studies, etc. But the interesting and telling part deals with a live CLL patient, who was administered Rituxan at the standard dose, once a week for four weeks. His blood was analyzed for Complement proteins before and after each infusion of Rituxan. Prior to the first administration, his complement level was normal. During infusion, it was clearly seen that Rituxan homed in on the CD20 positive B-cells and that just like it is supposed to happen, complement proteins, in turn, zeroed in on the CLL cell with the CD20-Rituxan bond (the antigen-antibody pair), to begin the process of destroying the whole shebang. As you would expect, at the end of the infusion, much of the available supply of the Complement proteins in the blood had been used up. There was a five-fold decrease in the concentration of these essential proteins by the end of just the first infusion! The level recovered somewhat by the next week, in time for the second infusion, but not all the way. By the time the fourth infusion rolled around, the crucial complement protein concentrations were down as much as ten-fold. Given time, the patient's body will produce more of these proteins. Three weeks after the last dose of Rituxan, the Complement levels were almost back up to normal.
If this discussion leaves you with the impression that Complement is pretty powerful and potent stuff, you are right. You know the saying: power corrupts and absolute power corrupts absolutely. Last thing you want is the very powerful complement running amuck, killing perfectly decent and good cells just because they looked kind of funny, in mimicry of the shoot first and ask questions later approach that seems to be popular these days. Without strong and powerful safeguards against the ravages of complement, our bodies will be reduced to bloody pulp in no time at all. Most cells express markers that protect them against Complement. Simply put, these markers deflect the killing power of complement, a shield against this type of 'friendly fire" attack. The higher the expression of these anti-complement markers, the more protected the cell.
ASH2003 Abstract # 2256:
Complement (C) Activation Is Required for Rituximab (RTX) Mediated Killing of CD20 Positive Cells, and a High Tumor Burden May Decrease Therapeutic Efficacy Due to C Depletion as a Consequence of Therapy. In Vitro and In Vivo Studies.
Ronald Taylor, Adam Kennedy, Michael Solga, Paul Beum, Patricia Foley, Margaret Lindorfer, Charles Hess, John Densmore.
Biochemistry, Universtiy of Virginia School of Medicine, Charlottesville, VA, USA; Comparative Medicine, Universtiy of Virginia School of Medicine, Charlottesville, VA, USA; Hematology/Oncology, Universtiy of Virginia School of Medicine, Charlottesville, VA
We investigated C activation, C3bi deposition, and cell killing when RTX was bound to Raji or DB cells, in serum (NHS) as a C source. In the presence of RTX and C, large numbers of C3bi molecules deposit per cell, and fluorescence microscopy revealed that C3bi co-localized with bound RTX. Both cell types are killed by RTX in the presence of NHS, and use of mAb 3E7, specific for C3bi, enhanced killing. However, in the absence of NHS, little or no killing was demonstrable. RTX was infused into monkeys and we found that it rapidly bound to circulating B cells and activated C; 2 min after RTX infusion, C3bi was co-localized with RTX bound to the B cells. A similar pattern of co-localization of C3bi and RTX was obtained in vitro in opsonization experiments with blood samples taken from patients with B cell lymphomas.
A patient with CLL was treated with the standard 4 week course of RTX (Rituxan) therapy. Analyses of blood samples taken during this time revealed the following: Immediately after the first infusion, flow cytometry measurements and fluorescence microscopy indicated that RTX and deposited C3bi (Complement proteins) were associated with both B cells and cellular debris, and a high degree of co-localization of C3bi with RTX was evident. After the first infusion, CH50 assays demonstrated that the patient's C titer (Complement level), normal before treatment, had been substantially reduced (~ 5 fold). Although C levels were partially restored before the second infusion, the same pattern of C depletion occurred after this infusion, and by week three and four the patient's C levels were reduced ~ 10-fold, but returned to baseline 3 weeks later. We found, however, that in vitro supplementation of the C-depleted sera with C component C2 markedly increased C activity, leading to levels that were at least 50% of the pre-therapy baseline. In vitro studies with RTX, B cell lines, and NHS gave rise to similar findings. That is, at high cell concentrations RTX-mediated C3bi deposition and killing is limited by serum C, and both of these activities can be enhanced by supplementation with purified human C2. Our results indicate that the primary mechanism of action of RTX in killing CD20 positive cells is mediated through C, and it is likely that the in vivo form of this mAb bound to target cells has covalently incorporated C3bi. We suggest that if an anti-tumor mAb such as RTX requires robust C activation for therapeutic efficacy, then insuring an adequate level of C activity in a patient, by supplementation with either fresh plasma or a purified C component such as C2, may provide an important approach for improving the therapeutic efficacy of a C-fixing mAb.
_________
Article from the Journal of Immunology
J Immunol. 2025 Mar 1;172(5):3280-8.
Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia.
Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, Densmore JJ, Williams ME, Taylor RP.
Department of Biochemistry and Molecular Genetics, University of Virginia School of Medicine, Charlottesville, VA 22908
Complement plays an important role in the immunotherapeutic action of the anti-CD20 mAb rituximab, and therefore we investigated whether complement might be the limiting factor in rituximab therapy. Our in vitro studies indicate that at high cell densities, binding of rituximab to human CD20(+) cells leads to loss of complement activity and consumption of component C2. Infusion of rituximab in chronic lymphocytic leukemia patients also depletes complement; sera of treated patients have reduced capacity to C3b opsonize and kill CD20(+) cells unless supplemented with normal serum or component C2. Initiation of rituximab infusion in chronic lymphocytic leukemia patients leads to rapid clearance of CD20(+) cells. However, substantial numbers of B cells, with significantly reduced levels of CD20, return to the bloodstream immediately after rituximab infusion. In addition, a mAb specific for the Fc region of rituximab does not bind to these recirculating cells, suggesting that the rituximab-opsonized cells were temporarily sequestered by the mononuclear phagocytic system, and then released back into the circulation after the rituximab-CD20 complexes were removed by phagocytic cells. Western blots provide additional evidence for this escape mechanism that appears to occur as a consequence of CD20 loss. Treatment paradigms to prevent this escape, such as use of engineered or alternative anti-CD20 mAbs, may allow for more effective immunotherapy of chronic lymphocytic leukemia.
PMID: 14978136
____________
So far we have discussed the tagging of the CLL cells by the pincers of the lobster. Now it is the turn of the lobster tail. In a properly engineered monoclonal antibody, once the lobster has grabbed the CLL cell, the CD20-Rituxan pair is gradually shepherded into a region of the cell surface called a lipid raft. Never mind what it is, think of it as the coral reef where all the locked-on lobsters congregate. Oh yes, each cancer cell has literally thousands of CD20 markers, so there is a huge concentration of these CD20-Rituxan pairs all in one spot. All of those lobster tails waving in the breeze is incredibly attractive to complement, and it contributes to the cell kill as we discussed above. The second important thing is that the lobster tails (called Fc region of the Rituxan molecule, if you must know) also attract every killer macrophage and neutrophil that happens to be around. These killer cells have receptors that mate exactly so with the lobster tails. Net result, the prey (the CLL cell) and the killers (macrophages, neutrophils, monocytes) are brought together, the lobster acting as the dating service. This is a close encounter of the fatal kind: the only reasonable outcome is that the CLL cell is killed. If you want to know the gory details of how exactly the cell is killed, you might want to read another article that describes the mayhem: Serial Killers - Up Close and Personal. Stephen King chain saw murder stories pale by comparison, and of course, there is no hint of the supernatural in all of this.
So, why is it that Rituxan works so well in NHL, but only so-so as a single agent in CLL? The problem is that we have fewer CD20 markers per CLL cell, so the tagging by the lobster hordes is not quite as efficient. Less jam-packed CD20-Rituxan pairs corralled nicely in the lipid rafts of the CLL cell means less complement is attracted to the spot which leads to less effective CDC. Less action on this front means fewer killer cells home in for the killing orgy: and therefore there is less ADCC. To make matters worse, some CLL cells learn how to evade the attention of complement, by sprouting inhibitory markers (CD55, CD59). It has also been suggested that Rituxan does not grab on quite tight enough to the CD20 marker, it lets go sometimes and drifts away. Sort of like Velcro patches that have seen better days, things don't quite stay stuck. Another angle is that perhaps the lobster tails are not quite sexy enough. Some jaded killer cells cruise on by without stopping to finish the job of cell kill.
Here then are some of the areas where things can be improved:
I made it a point to visit the poster presented by Genmab A/S at this year's ASH. If you are into pain and information overload like me, here is a link to the poster: HuMax-CD20 Monoclonal Antibody.
Genmab says their HuMax-CD20 is a fully human antibody which is effective at binding to the target CD20, and releases only very slowly from the target over time. By comparison, Rituxan is called "chimeric", part human and part mouse protein in the molecule. In February 2025, Genmab presented data from pre-clinical laboratory tests showing HuMax-CD20 appeared to kill tumor cells that were resistant to Rituxan. The data showed the antibody was highly effective in inducing complement mediated cytotoxicity (CDC). This newly invented lobster appears to be able to grab on and hold on to the CLL cell a lot better than Rituxan, as per the claims of GenMab, and this in turn means complement activity is better. All that commotion of complement coating the CLL cell (a process called opsonization) attracts local killer cells to the vicinity, which may also increase the efficiency of ADCC. Genmab says they have now collected data that appears to show Humax-CD20 is also effective in inducing ADCC. Below is the link to the GenMab site, which discusses the technology.
http://www.genmab.com/view_prod_subpage.asp?dokid=45&p=HuMax%2DCD20
From what I have read thus far on the GenMab site, I have not seen too much of an effort to make the tails of the lobsters more attractive, but I could be missing something. There are a couple of very smart folks in the U.K working on just that aspect of things, as a way of improving both Rituxan and Campath style new drugs, and I hope to bring good news on that front very soon. Below is a very recent 2025 article abstract that has a lot more details, (Dr.Van De Winkel JG is the chief science officer at GenMab) and a link where you can read the full text article for free.
Blood. 2025 Jun 1 [Epub ahead of print]
Blood Journal Article
Characterisation of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin's lymphomas.
Teeling JL, French RR, Cragg MS, Van Den Brakel J, Pluyter M, Huang H, Chan CH, Parren PW, Hack CE, Dechant M, Valerius T, Van De Winkel JG, Glennie MJ.
Genmab, Utrecht, The Netherlands.
Despite the rapid and widespread integration of chimeric CD20 mAb, rituximab, into the management of non-Hodgkin's lymphoma, its efficacy remains variable and often modest when used as a single agent. To develop more potent reagents, human immunoglobulin-transgenic mice were used to generate a panel of IgG1kappa CD20 mAb. All reagents bound strongly to CD20+ cells and recruited mononuclear cells for the lysis of malignant B cells. However, two mAb, 2F2 and 7D8, were exceptionally active in complement-dependent cytotoxcity (CDC), being able to lyse a range of rituximab resistant targets, such as CD20-low CLL, in the presence of human plasma or un-fractionated blood. Further analysis showed that 2F2 and 7D8, like rituximab, redistributed CD20 into Triton X-100 insoluble regions of the plasma membrane, but that they had markedly slower slow off-rates. To determine whether off-rate influenced CDC, a non-complement activating F(ab')2 anti-human kappa reagent was used. This reagent markedly slowed the off-rate of rituximab and increased its CDC activity to that of 2F2 and 7D8. Thus, with increasing evidence that mAb therapeutic activity in vivo depends on complement activation, these new CD20 reagents with their slow off-rates and increased potency in CDC hold considerable promise for improved clinical activity.
PMID: 15172969
____________
You have been good to stick with me thus far, trust me this is an over-simplified cartoon version of the actual science. Here is the link to the clinical trial sponsored by GenMab: http://www.clinicaltrials.gov/ct/show/NCT00093314.
As you can see, the inclusion criteria are pretty good. They are actually are looking for CLL patients so you don't have to try and sneak in as an SLL/CLL patient. The only condition that might cause a problem for some folks on the maintenance regimen of Rituxan is that you have to have been on the wagon for at least 6 months, no Rituxan or Campath, before you can get this new monoclonal. Of course, you are out of luck if you are pregnant or breast feeding. That seems to be an almost mandatory disqualification these days. The trial is offered at two sites, at the University of Iowa as well as at Ohio State University. The principal investigator at Ohio State is none other than our favorite, John Byrd. This clinical trial has not been recruiting for any great length of time. My bet is it has not been open for more than a couple of months. But I do not know how many slots they have open and I get the feeling they will be filled pretty quickly. So, if you are in the market for a potentially improved version of Rituxan as single agent, you had better get on the phone and contact these folks. The link above gives the contact information.
One last item and I will shut up. If you do succeed in registering for this clinical trial, remember where you heard about it, remember your friends at CLL Topics. You have an open invitation to write about your experiences with this interesting new drug, and we will publish you on our Patients' Corner or as a Case Study. Total anonymity guaranteed, you can make up a name and persona .for yourself. Here is your one chance to be a blond bombshell, if that is your fantasy.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2025-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
