 |
||||||||||
Date: November 2, 2024
by Chaya Venkat
We are all aware that one of the problems with CLL cells is that they do not die quietly on command, like all good little normal cells. Normal cells have a finite life and when their usefulness is over they obediently commit the cellular equivalent of suicide in the interest of the greater good of the community. Not so CLL cells. The "Bcl-2" family of proteins is the key that controls "apoptosis" (cellular suicide), and CLL cells have an extra helping of specific Bcl-2 proteins that makes them deaf to suicide commands. This makes Bcl-2 a very tempting target for therapy. If we can turn off this protein and its live-at-all-cost message, CLL cells will become a lot easier to kill.
A number of you have written asking about two Phase-I trials, involving agents called "AT-101" and "GX15-070". (Don’t you wish these new drugs had more memorable names? Kind of hard to remember variations of the alpha-numeric soup.) Both of these drugs target Bcl-2. The AT-101 trial has been announced by the CLL Research Consortium and it is apparently recruiting patients at UCSD and elsewhere. GX15-070 is recruiting patients at Georgetown and possibly other locations as well. I don’t have a whole lot of information about either of these compounds, but perhaps I should let you have what I know right now, and give you updates as we go along.
Another drug that targets Bcl-2 but has a lot longer track record is "Genasense", manufactured by Genta. Since all three drugs are designed to attack the same target, I thought I would include an update on Genasense as well in this article. Independent of how the new trials of AT-101 and GX-15-070 work out, or how Genasense progresses in future trials, Bcl-2 is an important target and you can bet we will be hearing a lot more about drugs that target this important marker in the years to come. I think it is important for us to understand some of the background, get a handle on the risks and rewards involved in going after this target. Did I say risks? You bet. The era of risk-free cancer therapy has not arrived yet.
 "Apoptosis"
is the natural pathway of cellular suicide by which the body culls out old,
damaged and defunct cells. Normal cells obey the suicide signals given by the body and die
on command. Not so cancer cells. This defect in obeying death signals is one of
the hallmarks of CLL. Until recently, failure to die appropriately was thought
to be the only mechanism by which the CLL cells accumulated in the body. More
recent work from some of our expert centers has demonstrated that CLL cells also
proliferate, and this in addition to their reluctance to die on command,
contributes to their accumulation in the body. Genasense, AT-101 and GX15-070
all focus on the cell death part of the equation.
"Apoptosis"
is the natural pathway of cellular suicide by which the body culls out old,
damaged and defunct cells. Normal cells obey the suicide signals given by the body and die
on command. Not so cancer cells. This defect in obeying death signals is one of
the hallmarks of CLL. Until recently, failure to die appropriately was thought
to be the only mechanism by which the CLL cells accumulated in the body. More
recent work from some of our expert centers has demonstrated that CLL cells also
proliferate, and this in addition to their reluctance to die on command,
contributes to their accumulation in the body. Genasense, AT-101 and GX15-070
all focus on the cell death part of the equation.
Bcl-2 belongs to a family of proteins (the entire family is called Bcl-2 as well, making for some confusion.) Some members of this family favor cell death (pro-apoptosis proteins) and the others prevent cell death (anti-apoptosis). For those of you who like to figure out how things work, Bcl-2 is involved in the proper functioning of mitochondria, the power plants that are an essential part of each cell. Every living cell, with few exceptions, has a number of mitochondria embedded within it. In simple terms, mitochondria are essential for converting food and nutrients into the power that is needed by the cell to live and function.
But like most power plants, mitochondria also produce a lot of toxic waste. Using the analogy of a large metropolitan city, power production is essential to the function of the city. But the power plant needs to get rid of its garbage periodically and it is important that the garbage is put out only at regular intervals when there is a garbage pick-up scheduled. Spewing out the toxic garbage into residential neighborhoods without control is a sure way to poison the city and kill it. Just imagine New York City on a hot summer day, when the garbage men are on strike. You get the picture.
The membrane surrounding a mitochondrion is akin to a ten foot high wall with barbed wire on top surrounding the power plant. There is a door in this wall, kept under strict lock and key. This door, called the permeability pore, is one of the few ways in which the toxic contents of the power plant can get out into the surrounding countryside. This door is controlled by the Bcl-2 family of proteins. Some of these proteins (such as Bax and Bak) are used to keep the door propped open, allowing free transport of the toxins ("cytochrome c") from inside the mitochondria into the cellular surroundings. Bcl-2, on the other hand, is the protein that keeps the door shut tight. A careful balance between the proteins that keep the door open and those that keep it shut is needed for proper control in a healthy cell. If the door is open all the time, healthy cells die sooner than they should, poisoned from the inside out. If it is kept too tightly shut, diseased and cancerous cells can avoid death signals and go on living well beyond their time. The over-expression of suicide preventing Bcl-2 has been observed in a wide range of cancers, including breast, lung, prostate and colon cancers. CLL cells typically over-express Bcl-2, and this is particularly true of poor prognosis varieties of CLL as well as refractory cases.
Many well known chemotherapy drugs attack the mitochondria as the point where cancer cells are most vulnerable, pretty much the way one would expect terrorists to target nuclear plants in close proximity to large towns. Chemotherapy drugs attempt to break down the defenses of the mitochondria and force their toxic contents to spew forth and poisoning the cell from the inside. Bcl-2 makes that job harder, since it tries to keep the door in the wall surrounding the power plant shut tight. In other words, cancer cells that have an abnormally high expression of Bcl-2 are less likely to respond to chemotherapy. If we can neutralize the protective effects of high levels of Bcl-2, it becomes that much easier to target the mitochondria, and thereby kill the cancer cell. Once the internal contents of the mitochondria are forced to spill out, the cell is effectively poisoned and soon dies. I hope this little vignette has given you a quick peek into how cells work and why Bcl-2 is an important target. You may also wish to read an earlier article we had on the subject of mitochondria, what makes them tick and how we can use that information in developing better cancer therapies (Target Mitochondrion).
Leuk Lymphoma. 2024 Apr;44(4):563-74.
Mitochondria as a target for inducing death of malignant hematopoietic cells.
Solary E, Bettaieb A, Dubrez-Daloz L, Corcos L.
INSERM U517, IFR 100, 7 boulevard Jeanne d'Arc, 21000 Dijon, France.
Mitochondria plays a central role in apoptotic cell death. The intermembrane space of mitochondria contains a number of soluble molecules whose release from the organelle to the cytosol or the nucleus induces cell death. Thus, molecules that directly trigger mitochondria membrane permeabilisation are efficient cytotoxic drugs. Mitochondria is one of the cellular targets for commonly used epipodophyllotoxins, adenine deoxynucleoside analogs and taxanes as well as recently developped agents such as the pentacyclic triterpene betulinic acid and the lymphotoxic agent FTY720. Most informations on anthracyclines point to the mitochondrial membrane as the main target of cardiotoxicity. Mitochondria is also a target for arsenite trioxide, an old cytotoxic agent recently used for treating acute promyelocytic leukemia, lonidamine, a dichlorinated derivative of indazole-3-carboxylic acid developped as a chemosensitizer, the retinoic acid receptor gamma activator CD437 and nitric oxide (NO). Recently, cytotoxic drugs have been specifically designed to directly affect the mitochondrial function. These include the positively charged alpha-helical peptides, which are attracted to and disrupt the negatively charged mitochondrial membrane, thus inducing mammalian cell apoptosis when targeted intracellularly. Various strategies have been proposed also to directly inhibit Bcl-2 and related anti-apoptotic proteins, including antisense oligonucleotides (e.g. Genasense, currently tested in phase III trials), small molecules that mimic the BH3 dimerization domain of these proteins and kinase inhibitors. Ligands of the mitochondrial benzodiazepine receptor such as the isoquinolone carboxamide derivative PK11195 also overcome the membrane-stabilizing effect of Bcl-2, whereas the adenosine nucleotide translocator (ANT) and the mitochondrial DNA are two other potential cellular targets for cytotoxic agents. Potentially, new compounds directly targeting the mitochondria may be useful in treating hematological malignancies. The challenge is now to selectively target these mitochondria permeabilizing agents to malignant cells. This review briefly summarizes the role of the mitochondria in cell death and describes these various strategies for targeting the mitochondria to induce apoptosis.
PMID: 12769332
______________
Genasense is made by Genta Corporation. Here is how it works. You know that the genetic blueprint of each cell in your body is stored in strands of DNA, in the nucleus of the cell. Think of DNA as the how-to instruction books in the local library. Continuing our analogy, messenger RNA or "mRNA" is similar to the handyman that consults the DNA instruction manuals to build exactly the right kind of proteins for the proper functioning of the body. What happens when we tie up the handyman so that he is unable to do much of anything? To be even more specific, what happens when we tie up the handyman in such a way that he is prevented from making one specific protein?
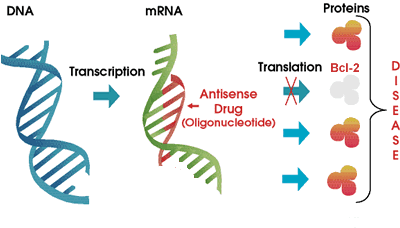 That is pretty much how Genasense is thought to work. This drug
is a type of "antisense" molecule, a small strand of DNA that is exactly the
opposite of a section of the mRNA molecule. It is a small strand only, gumming
up the works of the mRNA only at the point that it is needed to make the Bcl-2
protein. In an ideal world, the cellular mRNA is hog-tied only to the extent
that it cannot make Bcl-2, but it is free and welcome to make all of the other
proteins. Also in an ideal world, since cancer cells are so much more dependent
on Bcl-2 to keep them living longer than they should, controlling and reducing
the level of production of Bcl-2 would hurt cancer cells a whole lot more than
it would hurt regular cells. This is a neat concept, and I have to admit I had
high hopes for Genasense when it made its initial debut several years ago. I
liked the specificity of targeting only Bcl-2, a vulnerable point for
cancer cells. Early hype notwithstanding, we reported in an earlier article
that Genasense has been a disappointment in CLL clinical trials
(ASH
2004 Highlights).
That is pretty much how Genasense is thought to work. This drug
is a type of "antisense" molecule, a small strand of DNA that is exactly the
opposite of a section of the mRNA molecule. It is a small strand only, gumming
up the works of the mRNA only at the point that it is needed to make the Bcl-2
protein. In an ideal world, the cellular mRNA is hog-tied only to the extent
that it cannot make Bcl-2, but it is free and welcome to make all of the other
proteins. Also in an ideal world, since cancer cells are so much more dependent
on Bcl-2 to keep them living longer than they should, controlling and reducing
the level of production of Bcl-2 would hurt cancer cells a whole lot more than
it would hurt regular cells. This is a neat concept, and I have to admit I had
high hopes for Genasense when it made its initial debut several years ago. I
liked the specificity of targeting only Bcl-2, a vulnerable point for
cancer cells. Early hype notwithstanding, we reported in an earlier article
that Genasense has been a disappointment in CLL clinical trials
(ASH
2004 Highlights).
A couple weeks ago I had a long telephone conference call with Dr. Raymond Warrell, CEO of Genta. I will certainly give the company high marks for making this effort to reach out to our patient community. Some of you may have heard anecdotal accounts of a patient volunteer who felt left in the lurch back in the early years after participating in a Genasense trial. But we are always willing to look to the future, build good working relationships with companies that are willing to work with us and willing to develop patient-friendly protocols. As a result of this conversation, I got a better understanding of their most recent clinical trial which opened in 2024, a combination of Genasense with Rituxan and fludarabine. Think of this as your standard combination of Rituxan + fludarabine pioneered at Ohio State ( RF Therapy Clinical Trial, Fludarabine Monotherapy Is No Longer the Gold Standard) with the added kick of Genasense. This is a single arm, open label trial. In other words, every one who participates in the trial will get the same combination of all three drugs, there is no control group.
This trial is looking to recruit about 80 patients, and it is currently recruiting patients at Georgetown university, Long Island Jewish hospital and Roswell park. Both chemo-naïve and previously treated patients are eligible. The only substantive exclusion criteria I see are autoimmune disease and prior bone marrow transplant. The link below will take you to the public announcement of the trial, and the PDF gives a bit more about the study outline including contact information if you are interested.
http://clinicaltrials.gov/show/NCT00078234
Genasense Study Outline (PDF document).
My prior article (ASH 2004 Highlights) expressed disappointment based on the results Genta reported at the ASH2004 meeting, regarding an earlier Phase-3 clinical trial. Dr. Kanti Rai reported the results of an extensive double arm study of Genasense + fludarabine + cyclophosphamide, versus just the two chemo drugs without addition of Genasense. 241 refractory / relapsed patients participated in this study. All of them had at least one prior regimen of fludarabine going into this clinical trial. During my conversation with Genta, I was able to get an update of this study, and I would like to share the high lights with you. You can look at more of the details of the results and analysis of this study in Genta's slide presentation (PowerPoint document: slow download).
1. The "primary endpoint" of this study was the number of patients who got a CR (complete response) or nPR (nodular partial response) as defined by the NCI criteria. Unfortunately, there were not a whole lot of them in either arm of the study. The G+FC group had 17% CR + nPR, while the control group got 8% CR + nPR, this is borderline statistical significance (p = 0.016). This difference went away if we threw in partial responders as well.
2. The folks who got CR or nPR responses in either group were tracked for 32 months. Only 5 out of the 20 patients who got CR or nPR in the Genasense group relapsed, while 6 out of the 8 patients in the control group relapsed. But when we look at all 241 patients who participated in this trial, there was no difference in the time to progression between the two groups.
3. There was no increase in hematological toxicity as a result of adding Genasense to F + C. Thank Providence for small mercies.
There is a hint here in the data that if patients went into the G + F + C combo when they were still in pretty good shape, not too refractory or carrying the burden of too many prior therapies in their past, they are more likely to benefit from Genasense. And the CR or nPR remissions obtained for such patients may prove to be more lasting than those in the control group. However, this study has only a total of 28 patients out of the whole lot of 241 patients that got CR or nPR responses. Slicing and dicing data on smaller subsets of patients takes away a great deal of the confidence one gets from large studies such as this. Statistical differences based on 28 patients is not the same thing as differences seen on the whole group. I would have dearly liked to see a significant difference in the overall time to progression between the 120 patients who got the G + C + F versus the 121 patients in the control group. Unfortunately, that was not the case.
In light of these results, design of Genta’s new clinical trial of G + R + F makes sense. First, Rituxan seems to make everything work better. Why not use it as one of the drug combo, and drop cyclophosphamide from the mix? Also, this new trial includes chemo-naïve as well as pre-treated patients. If indeed earlier-stage, chemo-naïve patients and patients in better shape are likely to get more of a benefit from Genasense, that effect should become obvious when we compare chemo-naïve versus pretreated patients inducted into this study. While the new study is single arm (in other words, there is no control group), hopefully there is enough information out there about the RF combo, and we can compare the results of the G+RF versus those historical results of RF. Not quite the same thing as a formal phase-3 study with a built in control group, but it will have to do for now.
During our conversation I raised the issue of clinical trials combining Genasense with novel low toxicity adjuvants. EGCG and fenretinide come to mind. If the idea is to use Genasense in earlier stage patients, why not go all the way and use this drug in less toxic combinations? The RF combination is hardly a walk in the park. Add on top of that the hassles and out of pocket expenses of participating in a clinical trial, and we are setting up substantial barriers for patients. I can get pretty long-winded and passionate when it comes to lobbying for more patient friendly clinical trials. I think I got my message through. Here is direct quote from the CEO of Genta:
"We are keenly aware of the daily challenges faced by patients with devastating diseases, who then must also cope with the difficulties of maneuvering a complicated health care system, as well as the substantial additional burden posed by participating in clinical trials. As a company, we want to make our trials as accessible to patients as possible. Moreover, as a core strategy, we also want to develop programs with Genasense that increase patient convenience and minimize side-effects. For example, while our last Phase 3 study in CLL required an IV infusion, this quarter we will be starting a program that evaluates Genasense treatment by subcutaneous injection, like an insulin shot. That method will obviate requirements (and complications) of catheters and reduce the number of visits to medical clinics. Next, we are testing Genasense in combination with other drugs and regimens that are much less intensive and immunosuppressive than Flu/Cy in CLL. Lastly, we are developing "next-generation" Genasense compounds that could further reduce Genasense therapy to a once-per-cycle treatment."
Encouraging words. We look forward to better therapy options for our patient community in the years to come.
Editor's Note: Below are comments from Dr. Ray Warrell, CEO of Genta, published here with his explicit permission. Dr. Warrell points out, and we concur, the Phase-3 double blinded and controlled clinical trial of Genasense with F+C is an impressive endeavor. Multi-center trials such as this involving hundreds of patients are both expensive and hard to do, but nevertheless essential if we are to develop strong foundations for future therapeutic regimens. Genta is to be congratulated for their efforts. His comment that over 2,500 patients have been treated with Genasense without observing cardio-toxicity is worth noting, and gives reassurance to the patients considering participation in future trials.
Dr. Worrell's Comments: ... The CLL Topics article is very authoritative and extremely well-written. Please extend my congratulations to Venkat. I don't have any corrections whatsoever. I'm sure Venkat will understand that we're considerably more enthusiastic about the benefits of Genasense for patients for some of the following reasons.
- We took the time and very high expense to conduct a multiyear, prospective, randomized trial. Comparisons/extrapolations to patients in any other trials are extremely hazardous. For example, all responses in the Genasense study were refereed blindly and independently for both clinical response and radiology. In the Genasense study, CT scans were part of the independent review for primary endpoint response, which is not usually the case with studies that have used NCI-Working Group criteria.
- Our patients were heavily pretreated. All had received fludarabine, one-third had received rituximab, and about 10% had received Campath. Flu/Cy is considered the best available therapy for these patients; moreover, that was the regimen they were going to receive from their physicians. So, as a patient facing treatment with Flu/Cy, the question would be: "Does the addition of Genasense make a difference if I'm getting Flu/Cy, weighing benefits and risks?"
For the first time in any Phase 3 study, this study proposed a very stringent endpoint, namely the complete (i.e., not partial) eradication of all signs and symptoms of CLL. As noted, the percent of patients who were able to achieve this level of high-quality response was significantly superior when Genasense was added. Moreover, if you achieved this level of response, your likelihood of remaining in a completely disease-free state was significantly higher if Genasense was added to the regimen.- I have no information regarding safety of the other drugs mentioned. Bcl-2 is expressed in the heart, as well as many other tissues; however, expression does not connote criticality of function. For example, several strains of mice have been genetically engineered as "Bcl-2 knockouts", meaning they have no Bcl-2 at all in any tissue. The hearts, lungs, livers, brains, (etc.) of these animals are all normal. What's observed is profound loss of lymphocytes and melanocytes. (Hence, our keen interest in CLL and melanoma.)
We have completed careful animal toxicology studies with Genasense over prolonged periods and have not observed cardiotoxicity. We have also treated more than 2,500 patients in Genasense trials that have now extended more than 10 years without observing cardiotoxicity. Thus, the reason we no longer monitor cardiac function (as opposed to other organ function) is that we already have the data that demonstrates the safety. One of the key advantages of using antisense is its extremely high selectivity, which minimizes "off-target" effects. With other drugs, particularly small molecules, there are inevitably effects that have nothing whatsoever to do with modulation of Bcl-2. Thus, demonstrating that some agent inhibits Bcl-2 as one function in no way should suggest that's the only function.
Hope the above thoughts are helpful for the discussion.
Ray
________________
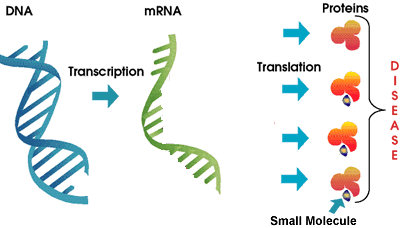
As we discussed above, Genasense is a relatively large molecule that interferes with the work of the mRNA in the production of a specific Bcl-2 protein. In contrast, the GeminX drug GX15-070 is a small molecule drug that blocks the function of a number of Bcl-2 family proteins already in place. Some researchers believe that inhibition of a single member of the Bcl-2 family is unlikely to result in a therapeutic effect. Instead, inhibition of all anti-apoptosis proteins in the family may be needed to make the cell death program to proceed on command. GX15-070 is designed to target the entire Bcl-2 family of anti-apoptosis proteins instead of a single member, by exploiting the structural similarity of Bcl-2 proteins. Besides being a small molecule compared to Genta’s Genasense, this is the other major difference between the two approaches. It remains to be seen if this new approach plays out better in CLL. Does the shotgun approach targeting many members of the Bcl-2 family have a better chance of success? Or will this prove to be unduly wide focus and therefore more likely to have unanticipated side effects? Only time and a well conducted (and promptly reported!) clinical trial will resolve this question.
GX-15-070 is made by GeminX , a
Canadian company focused on small molecule drugs targeting Bcl-2 and p53
pathways. The trial that is presently recruiting patients is an early stage
Phase 1/2 trial. It is an open-label, dose-escalation single agent study
designed to evaluate the safety and establish the correct dosage for GX15-070.
Unfortunately, the trial is not announced on public sites such as
www.clinicaltrials.gov,
a serious omission from the perspective of patients. I
have a phone call in to the company, to try and see if I can get them to correct
this problem. Clinical trial announcements and pertinent information should be
available to all patients, not just the select few who happen to be patients at
the cancer centers testing the new drugs. Lack of transparency in patient
recruitment can lead to concerns about "cherry picking", biased results stemming
from biased recruitment. I will let you know if the company gets back to me.
Fortunately, we have our own sources of information, and I will be discussing
below the fine details of this clinical trial protocol. Last I heard, several
patients have already signed up for this trial at UCSD and Georgetown.
You will find the patient information sheet for this trial at the link below:
GX15-070 Patient Information
- Approximately 90 CLL patients are to be recruited for this trial between UCSD and Georgetown University, an ambitious target for such an early phase trial. My guess, this trial will be open for recruitment for a while longer, unlikely to be sold out right away. I think you will be able to take your time to check things out before you sign on the dotted line.
- For a change, this trial is restricted to CLL patients only.
- One of the major exclusion factors is any kind of history of seizures. I get the feeling this is an important exclusion criterion. I will have more to say about this in the editorial section of this article.
- GX15-070 will be administered once every three weeks to patients with CLL who have previously been treated with an alkylating agent and fludarabine. In other words, it very properly rules out chemo-naïve patients.
- The protocol says it in blunt terms, this is an experimental drug. The major goal of this clinical trial is to establish the right dosage of the drug, figure out how long it lasts in your body and how it works. While the pre-clinical work with cell lines and lab animals has shown that it works by inhibition of the Bcl-2 family of proteins, this has yet to be shown to be the case in human beings.
- As with all early phase trials, the major intent of this trial is not to provide specific therapeutic benefit to the participants. The acknowledged agenda here is to learn more about how this drug works, so that future generations of patients will benefit from the lessons learned by conducting this trial. I cannot emphasize too much the importance of this statement; it is not one to be disregarded by you or the researchers. All too often patients see this as a "CYA" statement, given with a wink and a nod. Not so, folks. You have to be very clear in your understanding of this point, and be sure of your own motivations for participating in such a trial. Our knowledge of CLL and how to treat it progresses by means of clinical trials such as this one and the generous volunteers who participate in them. But if you are signing up because this is the latest and greatest thing to come along (and you expect to be cured by it), I suggest you buy a lottery ticket instead.
- The study protocol involves quite a bit of time commitment. The GeminX drug is given as an intravenous infusion over an hour or so and there are no planned hospital stays. But you can plan on many visits to the research center for sample collection and monitoring.
- You should be prepared for quite a few blood samples, so that the researchers can figure out what is happening. There are also several chest X-rays, as well as one or more bone marrow biopsies. I did not see any specific mention of monitoring cardiac function, and this is one of the points that I wanted to clarify with the company. More about this point in the editorial section.
"GX15-070 is the first small molecule in human trials that targets Bcl-2 proteins, which are involved in many types of cancers," explained Dr. Thomas Kipps, head of the CLL Research Consortium. "In particular, some members of the Bcl-2 family are thought to play a significant role in the etiology of CLL, making this disease a logical choice as a development indication. I look forward to evaluating the compound's potential in this patient group." "GX15-070 (GeminX) is currently under clinical evaluation at UCSD and several patients have been enrolled in this clinical trial. GX15-070 has shown activity in a variety of in vitro cell-death assays (NSCLC, prostate, colon, and cervical) as well as in several in vivo xenograft and syngeneic animal models (prostate, colon, cervical, and mammary). Furthermore, we have demonstrated that GX15-070 induces apoptosis in clinical samples from CLL patients."
These are optimistic statements - and it is a good thing to put your best foot forward when starting out on a new adventure. But as the guys who will be looking at the sharp end of the infusion needle, you should be aware that this is truly an early phase trial. Much of the prior information comes from cell lines in lab glassware, or in animal studies. Unlike Genasense or AT-101 reviewed below, I was not able to find much information about this drug in any human trials. The recent abstract below from the Hutch underlines the importance of understanding these issues.
Biochim Biophys Acta. 2024 Dec 10;1705(1):43-51.
Promises and challenges of targeting Bcl-2 anti-apoptotic proteins for cancer therapy.
O'Neill J, Manion M, Schwartz P, Hockenbery DM.
Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N., D2-190, PO Box 19024, Seattle, WA
Cancer cells with elevated levels of BCL-2 and related survival proteins are broadly resistant to cytotoxic agents. Antisense oligodeoxynucleotides, and more recently small molecule ligands for BCL-2 and BCL-XL, are directly cytotoxic or synergistic with standard cytotoxic agents, and in some cases, may demonstrate selectivity for tumor cells. The usual issues for rational drug discovery are writ large upon BCL-2-targeted therapeutics. The molecular functions of BCL-2 are not well understood, such that validation of cytotoxic mechanisms related to BCL-2 as well as identification of surrogate markers for BCL-2 function are significant obstacles for drug development. Despite these problems, a substantial number of small molecules that bind to BCL-2 or BCL-XL are now available for pre-clinical testing; in turn, basic studies with these reagents should yield new insights about optimal strategies to disrupt BCL-2 survival functions.
PMID: 15585172
______________
|
Gossypol is a natural compound derived from cotton seeds. In the last century it was researched in China as a male contraceptive drug. But it never quite captured the imagination of guys, possibly because it was not entirely free of side effects (see below for some of the potential side effects). More recently, gossypol and its analogs have seen renewed interest, as potential cancer drugs. The reason for this is that gossypol serves as a small molecule inhibitor of the function of Bcl-2 and Bcl-Xl proteins. We have discussed the role of Bcl-2 as an anti-death protein, allowing cancer cells to live longer than they should. Bcl-Xl is another member of the family, it too is implicated in the ability of cancer cells to ignore suicide commands and hang around much longer than they should. |
AT-101 has been developed by Ascenta Therapeutics. It is a derivative of gossypol. The exact modification of gossypol needed to tweak it to this form is likely to be a closely guarded company secret. I am basing my comments on the assumption that AT-101 is likely to behave similarly to the parent compound gossypol. At the bottom of this section are some interesting abstracts, I particularly recommend the first one from the Burnham Institute. In fact, if you are a glutton for detail like me, write to us and we will be happy to help you locate the full text copy of this article.
I have obtained a copy of the 12 page patient information and consent form for this trial. For those of you who like your information in cheat-sheet format, here is the skinny on this trial:
- Approximately 30 early stage chemo naïve CLL patients are expected to be recruited for this study. UCSD is one of the centers, I am not sure if there is another center as well where this trial will be offered. I will look into it and get back to you.
- The aim of this early phase study is to determine the right dosage of the drug, to look for potential adverse effects, study how long the drug lasts in your system and last, but not least, see if does any good in reducing CLL tumor burden.
- I am pleased to report that the researchers plan to do detailed blood work as well as EKGs to check for heart function, ahead of recruiting patients for the trial. Chest X-rays, CT / MRI scans and bone marrow biopsies will be done as needed. No mention is made of doing detailed prognostic testing such as IgVH gene mutation status, FISH, ZAP70, etc. But I would be surprised if these were not done in all new clinical trials. It would be interesting to see if the efficacy of AT-101 depends on the cytogenetics and prognostic classification of the patient. All too often, researchers do not release all the data that is collected in the clinical trial to the individual patient, considering much of it as purely for laboratory purposes. I leave it up to you to use your most charming and persuasive negotiating skills to spring the data, all the data, that pertains to you. It is your data, you should have full access to it.
- Another nice thing about this trial is that the drug comes in the form of pills, taken once a day, for a maximum of 12 weeks. Now, what can be easier than that?
- Remember, this is a dose escalation study. The first group of 3-6 patients will take 6 pills each day, adding up to 30 mg / day. This is the level at which AT-101 has been tested in other cancers. Patients in later groups may be asked to take as many as 15 pills each day, for a hefty 75 mg / day of the drug. Here comes that famous phrase — they are trying to establish the "maximum tolerable dose". Stop pretending to be so macho you guys. Sometimes I think this makes the good doctors think we are made of sterner stuff than is really the case.
- Common adverse effects are expected to be mostly GI tract related ones (nausea, diarrhea, abdominal pain and poor appetite). There may be skin rashes and mouth sores. Damage to the heart is mentioned as an unlikely risk, but one that has been seen in animal studies. Please see the editorial section of this article for my perspective on these adverse effects, what makes them likely suspects to monitor in Bcl-2 targeting therapies. And oh yes, it stands to reason that since gossypol’s first claim to fame is as a male contraceptive, it is unlikely that you will be able to father children during this period. No big deal, but you may be a bit less thrilled about loss of sex drive and possible testicle pain, listed as "less common" side effects.
- It is interesting that GX15-070 made a big deal about excluding patients with a history of neuronal problems such as seizures, but the AT-101 trial does not seem to have similar red flags.
- On the benefits side of the equation, the jury is out. You may get a good response, no response, or your CLL may even progress. But in my opinion, that is only one aspect and narrow definition of the "benefit" to participants. I would like to list one sure fire, guaranteed benefit from participating in this and other clinical trials. You will have the satisfaction of knowing you have done something very special to progress our understanding of CLL, and this is your personal gift to future generations. CLL is a familial disease. If we do not go the extra mile to get better therapy options developed for our kids and grandkids, who will?
This study has just kicked off. I doubt they have recruited more than a fraction of the total number they are looking for. Making a decision to participate or not is tricky in this case, since the recruitment is for early stage and chemo naïve patients, and the drug in question is such an experimental one. AT-101 has a little more track record than GX15-070. At least AT-101 has been used in other human trials for other cancers, and the parent compound has been used in China as a not-so-successful male contraceptive (see www.malecontraceptives.org) . On the other hand, GX15-070 is riding solely on pre-clinical animal studies. But be aware that AT-101 will be taken to significantly higher doses in later recruits for this trial, as much as 75 mg/day, while I believe most of the prior human trials went only as high as 30 mg/day. Higher drug dosage is often accompanied by more serious adverse effects.
Early stage patients have many options these days when considering front line therapy choices. That raises the bar — new drugs trying to break in have to meet a higher standard. Making decisions in the absence of clear information is very hard. I suppose if you have been blessed with cardiac or GI tract problems along with CLL, in your shoes I would think twice before signing up. To be honest, I get the feeling the researchers are not likely to recruit you for this trial if you had such risk factors.
At this point, I have only the experience of a single patient who is participating in this trial. Beware, this is strictly anecdotal information. It is impossible to predict how things will work out for you based solely on the experience of this one patient. Nevertheless, this is all we have at this point and I thought I would bring it to your attention (with the explicit permission of the individual involved). As I hear from more of you I will be able to flesh out the picture a bit more. Here are the patient’s comments, in his own words.
September 27, 2024
I have some encouraging early results on the Phase 1 trial for gossypol at UCSD. I am the third member of the trial and just started the dosage yesterday. It is too early for personal results; but, I thought folks would be interested to know that the first member has halved his counts in a matter of weeks (from 22 to 11), and the second member, in less time, has gone down 30% (from mid 30's to low 20's). He has also seen his spleen reduce from 6cm to 3cm and nodes return to normal. Since this is a dosage trial, no one was really expecting efficacy results this quickly.
My numbers going in are WBC at 55, 70% marrow infiltration, all other blood counts normal, spleen at 3cm and minimal nodal involvement. I express unusually high CD38 at 98%, am ZAP70 negative, mutated IgVH, and 12 Trisomy. I will post my early results in about 2 weeks. As a reminder - qualifications for the study are Stage 0 -2, W&W, no previous treatments, no current infections.
(Editor’s comment: This patient has a mixed bag of prognostic
indicators. Mutated IgVH is very good, as is negative ZAP70; but high CD38 and
Trisomy 12 are not so good. All in all, a medium risk patient, Bucket B resident
we think).
______________
October 10, 2024
I have been in the trial now for two weeks as the third member of
this phase 1 effort. I do not have enough current detail on them to speak of. My
counts since the first day of the trial 9/26 are essentially unchanged. Let’s
hope the numbers are down next time too. I will be tested again next week and
then it jumps out to every three weeks. I am not exhibiting any side effects or
symptoms at this time. I'll post again with updated results of course.
______________
October 25, 2024
I am sorry that this post isn't a little more timely. I guess I have been a little depressed about things lately and have sort of tried not to think about it too much. I actually missed a little work today because I haven't been myself. The nausea they said might kick in has and I am on Compazine now for that. My GI just isn’t normal now; and even though I keep trying to pretend I have an appetite, I really don’t.
Here are my latest WBC results - again unremarkable. My next appointment is 11/08 at UCSD.
Date |
WBC (X000) |
| 9/26 (start of protocol) | 51.0 |
| 9/27 | 52.1 |
| 10/03 | 55.4 |
| 10/10 | 46.1 |
| 10/17 | 51.4 |
My platelets have dropped below 200 for the first time ever and my HGB is still around 16. I really did not have high expectations for this trial since it is a Phase 1 so the results are not really what is depressing me. Maybe it's that after 8 months of this disease I am finally coming to some realization of what my quality of life will be like from now on. I hope I am not coming across as whining. I cant help the way I feel, and I truly admire those of you who have dealt so long with such obviously adverse treatments as chemo and the like. Maybe that is why I am down - I have only just begun this trip, and it aint much fun for sure. Still trying to keep the chin up though - I hate lookin' down.
______________
Chem Biol. 2024 Mar;11(3):389-95.
Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L).
Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, Kipps TJ, Reed JC, Pellecchia M.
The Burnham Institute, 10901 North Torrey Pines Road, La Jolla, CA
Antiapoptotic Bcl-2-family proteins Bcl-2 and Bcl-X(L) have been recently validated as drug discovery targets for cancer. Here, by using a combination of molecular modeling, NMR-based structural analysis, fluorescence polarization assays, and cell-based assays, we have designed and characterized a novel proapoptotic compound targeting these proteins. Our compound, Apogossypol, is capable of binding and inhibiting Bcl-2 and Bcl-X(L) with high affinity and induces apoptosis of tumor cell lines. Mechanistic studies on the action of our compound were also performed via confocal microscopy that provided real-time detection of the interaction with Bcl-X(L) in intact cells. Finally, preliminary data on cells freshly isolated from patients affected by chronic lymphocytic leukemia strongly suggest potential applications of Bcl-2 antagonists as chemosensitizers in cancer therapy.
PMID: 15123268
______________
Mol Cancer Ther. 2024 Jan;4(1):13-21.
Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(-)-gossypol] against diffuse large cell lymphoma.
Mohammad RM, Wang S, Aboukameel A, Chen B, Wu X, Chen J, Al-Katib A.
Department of Internal Medicine, Division of Hematology and Oncology, Karmanos Cancer Institute, Wayne State University School of Medicine, 724 HWCRC, 4100 John R. Street, Detroit, MI
Overexpression of Bcl-2/Bcl-X(L) protein has been observed in more than 80% of B-cell lymphomas. Diffuse large cell lymphoma (DLCL) is the most common subtype of non-Hodgkin's lymphoma. (-)-Gossypol, a natural product isolated from cottonseeds, was discovered as a potent small-molecule inhibitor of Bcl-2 and Bcl-X(L) proteins, with a Ki value in the nanomole per liter range for both. In vitro, (-)-gossypol showed significant growth inhibition effect against WSU-DLCL2 lymphoma cell line and fresh cells obtained from a lymphoma patient with no effect on normal peripheral blood lymphocytes. As expected (-)-gossypol induced complete cytochrome c release from mitochondria, increased caspases-3 and -9 activity, and caused apoptotic death without affecting protein levels of Bcl-2, Bcl-X(L), Bax, and Bak. The addition of cyclophosphamide-Adriamycin-vincristine-prednisolone (CHOP) regimen to lymphoma cells preexposed to (-)-gossypol enhanced killing significantly. The maximum tolerated dose of (-)-gossypol in severe combined immunodeficient (SCID) mice was 40 mg/kg for three i.v. injections when given alone and 20 mg/kg x 3 when given in combination with CHOP. Using WSU-DLCL2-SCID mouse xenograft model, the tumor growth inhibition, the tumor growth delay, and the log10 kill of mice treated with (-)-gossypol + CHOP were better than CHOP or (-)-gossypol alone. We conclude that adding Bcl-2/Bcl-X(L) small-molecule inhibitor to standard chemotherapy may prove an effective strategy in lymphoma therapy.
PMID: 15657349
______________
J Med Chem. 2024 Sep 25;46(20):4259-64.
Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting BCL-2 proteins.
Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M.
The Burnham Institute, 10901 North Torrey Pines Road, La Jolla, CA
Among the most promising chemopreventive agents, certain natural polyphenols have recently received a great deal of attention because of their demonstrated inhibitory activity against tumorigenesis. In view of their anticancer properties, these compounds also hold great promise as potential chemotherapeutic agents. However, to translate these chemopreventive agents into chemotherapeutic compounds, their exact mechanisms of action must be delineated. By using a multidisciplinary approach guided by modern nuclear magnetic resonance spectroscopy techniques, fluorescence polarization displacement assays, and cell-based assays, we have begun to unravel the mechanisms of actions of certain polyphenols such as Gossypol (a compound from cotton seed extracts) and Purpurogallin (a natural compound extracted from Quercus sp. nutgall) and their derivatives. Our findings suggest that these natural products bind and antagonize the antiapoptotic effects of B-cell lymphocyte/leukemia-2 (Bcl-2) family proteins such as Bcl-x(L). Our in vitro and in vivo data not only open a window of opportunities for the development of novel cancer treatments with these compounds but also provide structural information that can be used for the design and development of novel and more effective analogues.
PMID: 13678404
______________
Bcl-2 is a tempting target. It is what gives cancer cells their ability to avoid suicide signals given by the body, or even the more insistent invitation to do so by chemotherapy drugs.
If I were to make a guess, Bcl-2 targeting drugs such as these three will be more effective in combination with other chemotherapy drugs, not as single agents. Genasense has already embarked on this path, their latest clinical trial combines Genasense with Rituxan and fludarabine. I have no doubt that if they pass the test of these early phase trials, both GX-15-070 and At-101 will follow up with combination trials, pairing their versions of Bcl-2 blockers with other well known CLL drugs.
The holy grail of Bcl-2 targeting cancer treatments is to kill the vulnerable cancer cells (which have too many mitochondria and too much Bcl-2 to keep the mitochondria from spilling their toxic guts into the cell), and at the same time spare normal and healthy cells that have fewer mitochondria and less dependence on Bcl-2. Combining a Bcl-2 blocker with the killing power of conventional chemo-immunotherapy drugs such as R, F or C may be the classic one-two punch. That is the whole point of these early Phase-I trials, to determine the right dosage and right method of administration of the Bcl-2 blockers, just enough and not too much or too little, the perfect Goldilocks solution. Future trials will build on that information, combining the Bcl-2 blockers with a variety of other drugs.
Bear with me as I repeat a cliché that is nevertheless very true: there is no free lunch in cancer therapy. A limited number of non-lymphoid tissues also express high levels of Bcl-2, such as skin and intestine, long-lived stem cells, cells of the heart muscle and long-lived postmitotic cells such as neurons. Within these tissues Bcl-2's function as an antidote to apoptosis may confer necessary longevity to these cell lines. When we block Bcl-2 across the board, I wonder what happens to healthy cells that also express Bcl-2 for good and proper reasons. The three abstracts below are interesting reading. Protecting the function of Bcl-2 is presented as a possible way of preventing cardiac disease and neural cell death.
Does the improved efficiency of cancerous cell kill due to targeting Bcl-2 protein also translate into higher cell kill of healthy cells, such as those of the skin, intestine, heart or neurons? Our admittedly anecdotal account of one patient who is going through the AT-101 clinical trial identified intestinal problems among the adverse effects. Hmmm. I wonder if any of the other patients saw this too, and perhaps skin rashes or mouth sores.
One of the exclusion criteria for GX-15-070 is any kind of seizures. I wonder if this is because the researchers are being careful not to take on patients who already have any sort of neuronal complications. Double Hmmmm. Is there reason to expect peripheral neuropathy as a potential adverse effect?
Of the three trials, only the AT-101 trial protocol seems make an effort to monitor cardiac function, with an EKG. I would much rather all three trials used MUGA scans to measure the patients’ cardiac function. Since Bcl-2 is expressed by healthy cells of the heart, there may be cardiac risk as a result of drugs that target Bcl-2. I would think prudence calls for assessing cardiac function (LVEF: left ventricular ejection fraction) before and after, to make sure that there has been no additional damage done to cardiac tissue. Unlike cardiac disease caused by clogged arteries, loss of heart muscle is hard to treat, often irreversible.
Don’t get me wrong, most cancer drugs carry risks of one sort or another. No one in their right mind will indulge in cancer therapy, not if the alternative was anything less than a serious reduction in the duration and quality of life. We accept a certain amount of risk as the cost of keeping alive, keeping the cancer at bay. However, that does not mean we jump into therapy decisions without weighing risks versus rewards carefully, to the best of our ability. When it comes to clinical trials, it is even more important to have full transparency and candor on part of the researchers, since there are so many unknowns. Early phase trials are extremely important if we are to come up with better options down the road for all of us. Generous volunteers who decide to participate in these trials deserve nothing less than our respect and admiration, as well as our best efforts to protect them from anticipated and unknown risks.
J Cell Mol Med. 2024 Jul-Sep;9(3):609-22.
Don't lose heart - therapeutic value of apoptosis prevention in the treatment of cardiovascular disease.
Reeve JL, Duffy AM, O'brien T, Samali A.
Department of Biochemistry, National University of Ireland, Galway, Ireland.
Cardiovascular disease is a leading cause of death worldwide. Loss of function or death of cardiomyocytes is a major contributing factor to these diseases. Cell death in conditions such as heart failure and myocardial infarction is associated with apoptosis. Apoptotic pathways have been well studied in non-myocytes and it is thought that similar pathways exist in cardiomyocytes. These pathways include death initiated by ligation of membrane-bound death receptors, release of pro-apoptotic factors from mitochondria or stress at the endoplasmic reticulum. The key regulators of apoptosis include inhibitors of caspases (IAPs), the Bcl-2 family of proteins, growth factors, stress proteins, calcium and oxidants. The highly organized and predictive nature of apoptotic signaling means it is amenable to manipulation. A thorough understanding of the apoptotic process would facilitate intervention at the most suitable points, alleviating myocardium decline and dysfunction. This review summarizes the mechanisms underlying apoptosis and the mediators/regulators involved in these signaling pathways. We also discuss how the potential therapeutic value of these molecules could be harnessed.
PMID: 16202209
______________
Herz. 2024 Nov;27(7):662-8.
Oxidative stress and apoptosis in heart dysfunction.
Kumar D, Lou H, Singal PK.
Cardiovascular Research Group, Department of Medicine, University of Wisconsin, Madison, WI.
BACKGROUND: Heart disease is a major cause of morbidity and
mortality due to its complex pathogenesis. Myocyte cell loss through apoptosis
has been reported in a variety of cardiovascular disease conditions including
myocardial infarction (MI), ischemia-reperfusion injury, end-stage heart failure
and adriamycin cardiomyopathy.
POTENTIAL APOPTOTIC FACTORS AND THERAPEUTIC TARGET: The cell biology of the
apoptotic regulatory processes and the precise role of apoptosis in the
development of cardiac dysfunction need to be established. The upregulation of
proapoptic proteins, like Bax (a member of the Bcl-2 family proteins), caspases
and cytochrome c, with or without the downregulation of antiapoptic proteins,
including Bcl-2 (another member of the Bcl family), Akt and inhibitory apoptotic
proteins (IAPs), has been documented in different cardiac disease conditions.
However, mitogen-activated protein kinases (MAPKs) have been shown to be
involved in both apoptosis and cell survival. Apoptosis can be blocked by
inhibiting apoptotic regulatory pathways with caspase inhibitors and
overexpression of Bcl-2 and Akt. More recently, increased oxidative stress has
been shown to promote apoptosis, and antioxidants have been shown to inhibit
this process.
CONCLUSION: The ability of antioxidants to inhibit these apoptotic pathways has
raised the possibility of newer therapeutic treatment for various heart
diseases.
PMID: 12439637
______________
J Bioenerg Biomembr. 2024 Jun;37(3):179-90.
Inhibition of mitochondrial neural cell death pathways by protein transduction of bcl-2 family proteins.
Soane L, Fiskum G.
Department of Anesthesiology, School of Medicine, University of Maryland, Baltimore, Maryland.
Bcl-2 and other closely related members of the Bcl-2 family of proteins inhibit the death of neurons and many other cells in response to a wide variety of pathogenic stimuli. Bcl-2 inhibition of apoptosis is mediated by its binding to pro-apoptotic proteins, e.g., Bax and tBid, inhibition of their oligomerization, and thus inhibition of mitochondrial outer membrane pore formation, through which other pro-apoptotic proteins, e.g., cytochrome c, are released to the cytosol. Bcl-2 also exhibits an indirect antioxidant activity caused by a sub-toxic elevation of mitochondrial production of reactive oxygen species and a compensatory increase in expression of antioxidant gene products. While classic approaches to cytoprotection based on Bcl-2 family gene delivery have significant limitations, cellular protein transduction represents a new and exciting approach utilizing peptides and proteins as drugs with intracellular targets. The mechanism by which proteins with transduction domains are taken up by cells and delivered to their targets is controversial but usually involves endocytosis. The effectiveness of transduced proteins may therefore be limited by their release from endosomes into the cytosol.
PMID: 16167175
____________
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2024-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
