 |
||||||||||
Date: January 30, 2025
by Chaya Venkat
Other Articles on Revlimid:
Harvey's Journal Entry:
Revlimid - the Good, the Bad and the Ugly;
Topics Alert Number 176: Pushing Revlimid
Topics Alert Number 96: Revlimid — A Breakthrough?!?
I was a little surprised that so much of the buzz at ASH 2025 conference we attended recently was about Revlimid. Everyone was talking about it, and not just for Multiple Myeloma or MDS (myelodisplastic syndrome), diseases for which it is FDA approved therapy. There were two major papers describing use of Revlimid for relapsed / refractory CLL patients, one from M. D. Anderson and the other from Roswell Park. In keeping with our policy of full disclosure, I have to admit I had some misgivings about this drug. But I am enough of a scientist to change my mind if new evidence warrants it. So, here is the latest news on Revlimid and its potential value as a CLL drug — and as always, the editorial comments at the end of this review are my own take on this drug.
Below are the chemical structures of Revlimid and its parent drug, thalidomide. Even if you are not a chemist like me, it is easy to see that the two molecules are quite similar. Thalidomide is seeing a new resurgence, after disastrously unanticipated problems back in the 50’s. Some of you may remember the tragedy of the “thalidomide babies”. Thalidomide was prescribed to pregnant women in Europe (later followed in the USA) as a sedative and for reducing nausea. But it had much more potent effect on the developing fetus. We know now that thalidomide is a powerful suppressor of angiogenesis (for a discussion of angiogenesis, read Do You Like Drinking Green Tea?). Suppressing angiogenesis prevents the formation of new blood vessels, something that is essential for proper development of a growing fetus. As a consequence, some of the babies were born without limbs. The lax drug oversight that allowed this to happen was a huge scandal back then.
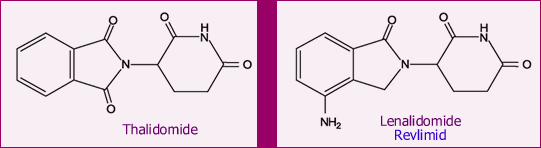
Today, thalidomide is a major drug used in the treatment of leprosy. Several analogs of thalidomide, variations on the theme as it were, are being studied for a variety of diseases. Lenalidomide (trade name Revlimid) is one of the most important, and it has made a name for itself in the treatment of multiple myeloma and MDS. As you would expect, this time around there is strict control on the use of this and all other thalidomide analogs, to make sure pregnant (or likely-to-get-pregnant) or breast-feeding women come nowhere near this drug. All patients who use this drug (men included, since we do not know if Revlimid is transmitted via semen) must swear they will use effective contraception to prevent any chance of pregnancy. This is not a major constraint in CLL, in my view, given the general age of our patients. When they dream of pitter-patter of little feet around the house, the majority of our members are thinking about grandchildren. Nevertheless, this is something to be aware of.
Before we get started, let us clarify one item that seems to confuse some of our members. Revlimid is the brand name of the drug distributed by Celgene Corporation. The generic name is lenalidomide. Both names mean the same thing, and you will see both names used interchangeably in the literature.
As I said above, two pivotal clinical trials using Revlimid were reported at the 2025 ASH annual meeting in Orlando. Given below is the abstract of the paper from Roswell Park. If you are considering Revlimid-based therapy, you may be well advised to read the full text of the article. Write to us, and we can help you locate it. We also have access to electronic versions of the detailed PowerPoint presentation made at the ASH, as well as the two posters that were on display. As always, write to us if you want to see these detailed and interesting presentations.
My review is pretty much focused on the Roswell Park paper. The M. D. Anderson paper presented broadly similar results. I would also like to mention here that Celgene Corporation has been very good about responding to our requests for additional information. I will be discussing their “RevAssist” program below, based on information the company provided. Many thanks to the medical information team at Celgene for initiating contact with CLL Topics, for an interesting telephone conference call and for making the detailed information available to us.
J Clin Oncol. 2025 Dec 1;24(34):5343-9. Epub 2025 Nov 6
Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study
Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, Porter CW, Goodrich DW, Bernstein ZP, Wallace P, Spaner D, Mohr A, Byrne C, Hernandez-Ilizaliturri F, Chrystal C, Starostik P, Czuczman MS.
Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY 14263
PURPOSE: Patients with relapsed or refractory chronic lymphocytic leukemia (CLL) have profound immune defects and limited treatment options. Given the dramatic activity of lenalidomide in other B-cell malignancies and its pleotropic immunomodulatory effects, we conducted a phase II trial of this agent in CLL.
PATIENTS AND METHODS: Patients with relapsed or refractory B-cell CLL (B-CLL) were eligible if they required treatment as per the National Cancer Institute Working Group 1996 guidelines. Lenalidomide was administered orally at 25 mg on days 1 through 21 of a 28-day cycle. Response was assessed after each cycle. Patients were to continue treatment until disease progression, unacceptable toxicity, or complete remission. Rituximab was added to lenalidomide on disease progression.
RESULTS: Forty-five patients were enrolled, with a median age of 64 years. Sixty-four percent of the patients had Rai stage III or IV disease, and 51% were refractory to fludarabine. The overall response rate was 47%, with 9% of the patients attaining a complete remission. Fatigue, thrombocytopenia, and neutropenia were the most common adverse effects noted in 83%, 78%, and 78% of the patients, respectively.
CONCLUSION: Lenalidomide is clinically active in patients with relapsed or refractory B-CLL. These findings are encouraging and warrant further investigation of this agent in the treatment of this disorder.
PMID: 17088571
____________
This is a single institution clinical trial — all patients were treated at Roswell Park. The majority of the patients were in Rai Stage 3-4, but earlier stage patients in Rai Stage 1-2 were also accepted if they needed therapy according to NCI criteria. Patients were not “naïve”, in the sense that they had to have been treated at least once previously and relapsed after that initial treatment. The rest of the inclusion criteria were pretty standard stuff: most patients would have had no trouble getting into this trial. As part of the pre-treatment evaluation, I was pleased to note that the majority (but not all) of the patients had a FISH test done. With everything we have learned in the past few years about the prognostic significance of chromosomal abnormalities in CLL, I see no excuse for any new clinical trials not to have FISH information on their patient cohorts. I wish the Roswell Park investigators had gone a little further and reported on the IgVH gene mutation status as well, but this was not the case. The most telling bit of information is to see how patients with poor prognostic 11q (ATM) deletion and the dreaded 17p (p53) deletion fared in this clinical trial.
| Characteristic | Value |
| Rai Stage 1-2 | 36% |
| Rai Stage 3-4 | 64% |
| Bulky Lymph Nodes | 22% |
| Refractory to Fludarabine | 51% |
| FISH Status | Available for 30 patients |
| 13q deletion | 16 out of 30 |
| Trisomy 12 | 4 out of 30 |
| "Normal" | 6 out of 30 |
| 11q deletion | 10 out of 30 |
| 17p deletion | 6 out of 30 |
| Total Number Recruited | 45 |
| Evaluable | 29 |
| Too early to tell | 4 |
| Consent Withdrawn | 5 |
|
Unable to Continue (due to toxicity) |
7 |
I think most of us would agree this is a tough crowd of patients. Just over half of them were fludarabine refractory and as we know all too well, that spells trouble. It is a pity they did not make FISH testing mandatory for all participants in the clinical trial but out of the 30 patients who had this test done, fully 16 had what I would call “Bucket C” (What Type of of CLL Do You Have?) abnormalities of 11q (ATM) and 17p (p53) deletions.
I was surprised by the large number of patients who dropped out of the trial after going through the trouble of recruitment and signing up for it. Out of the 45 patients recruited, only 29 are evaluable at this point. 5 patients withdrew consent, and 7 were too sick to continue. This is a higher percentage than I would have expected, and it speaks to the level of toxicity and side effects associated with this drug. We will be discussing this aspect of it in more detail, under the section detailing “Adverse Effects”.
In this single institution Phase-2 study, Revlimid was given orally (25 mg) once each day for 21 days, with 7 days off for good behavior. This 28 day cycle was repeated until the patient got a full PCR negative response, or the toxicity was bad enough to quit. If patients had CLL progress on them while on Revlimid, Rituxan was added as a secondary drug. This was also the case if Revlimid did not achieve better than merely stable disease over a period of 2 consecutive months. When Rituxan was used, it was given once a week at the “standard” dose of 375 mg/m2, for three weeks in the first Revlimid cycle, and for two weeks on each subsequent cycle.
Two of the patients recruited earlier on in the trial developed Tumor Lysis Syndrome. (TLS may be a potentially lethal complication seen in cancer patients undergoing chemotherapy. In simplified terms, TLS occurs when very large number of cancer cells are killed quickly, faster than the body can handle the cellular debris that is created by the dying cells. The body’s electrolyte levels go out of kilter (conditions called hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia) and this could lead to acute kidney failure and cardiac problems. You can read more about this important risk factor by clicking on this link: http://www.emedicine.com/med/topic2327.htm ). As a result of the observed TLS, the protocol was revised to include slow dose escalation of the Revlimid.
| Toxicity Grade | Grades 3-4 | All Grades |
| Hematological Toxicity | ||
| Thrombocytopenia | 45% | 78% |
| Neutropenia | 70% | 78% |
| Anemia | 18% | 63% |
| Other Toxicity | ||
| Fatigue | 10% | 83% |
| Flare Reaction | 8% | 58% |
| Tumor Lysis Syndrome | 5% | 5% |
| Pulmonary Embolism | 5% | 5% |
| Febrile Neutropenia | 15% | 15% |
| Infections | 5% | 23% |
The NCI Toxicity Criteria divides adverse effects into four grades, ranging from 1 through 4. It is important for us to know what the different grades mean. You can read all 72 pages of the official description by clicking on the link: NCI Toxicity Grade Definitions. Or you can read my much shorter version of it below:
Grade 1: Nothing much to write home about;
Grade 2: Definitely worth noting, but not enough to review your Will and last Testament;
Grade 3: I would say “uncle” at this stage, but then I am a wimpy patient advocate. You might be more macho than me;
Grade 4: Here is where you stop being brave, quit the stiff-upper-lip nonsense, and make sure you get good medical help.
In this trial, grades 3-4 level hematological toxicity were remarkably high, including thrombocytopenia (low platelets), neutropenia (low neutrophils) and anemia (low red blood cells). However, to be fair, many of these were relapsed and late Rai stage patients and many of them had thrombocytopenia to begin with. Some of these patients had an improvement in their platelet counts after the start of therapy, while thrombocytopenia got worse in others.
Grade 3-4 neutropenia was seen in a whopping 70% of the patients and six of them went on to develop febrile neutropenia. The protocol allowed the use of G-CSF (granulocyte colony stimulating factor; “Neupogen” and “Neulasta” are brand named G-CSF drugs) as needed. Below is a chart of the average neutrophil counts of all the patients in the study, over a period of 40 weeks. I would expect the periodic upward spikes are a result of Neupogen or Neulasta administration. But the improvements seem to be short lived after administration of these growth factors, since the general trend in average neutrophil counts is inexorably downwards aver the 40 weeks.
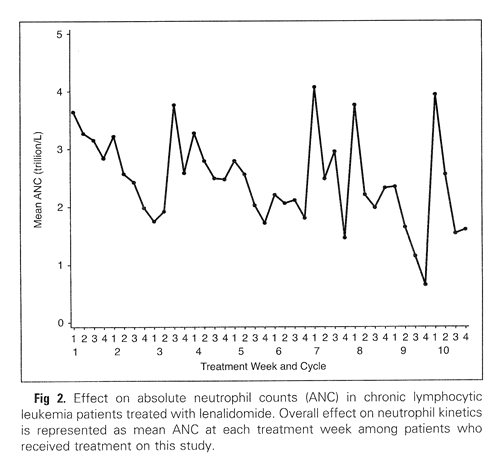
Neutrophil Counts Reported During Revlimid Therapy
(from Chanan-Khan, et al., JCO December 2025)
The flare reaction seen in more than half the patients deserves special mention. This is an effect that is not often seen in other therapy regimens and patients may not be familiar with it. The flare reaction typically sets in within one day of the very first dose of Revlimid and can last as long as 14 days. It is most common only in the very first cycle. The flare reaction is accompanied by swelling and tenderness of lymph nodes and spikes in the peripheral WBC numbers. Ibuprofen was sufficient to control the pain due to swollen and tender lymph nodes for some of the patients but a few patients needed oral morphine to bring the pain under control. The researchers could not tell ahead of time which patients were going to have more of a problem due to the flare reaction. I would not be surprised if the flare reaction was the major cause of the recorded patient withdrawal from the clinical study.
Tumor Lysis Syndrome is nothing to kid about. This can be life threatening, if not diagnosed and treated promptly. Two of the patients in this study developed TLS and one of them died subsequently due to congestive heart failure. This patient had prior history of cardiac disease and that may have contributed to his death. However, the message is clear: if you have prior history or risk of cardiac disease, you may want to think twice before you opt for Revlimid as the therapy of your choice. At the very least, you would want your doctors to be very aware of your prior history, so that they can monitor you with extra vigilance on this front.
Now we get to the “reward” side of the equation, the reason why any one would want to go through Revlimid therapy (or indeed any chemotherapy).
| Response | % | Number |
| Complete Response (CR) | 9 | 4 |
| Partial Response (PR) | 38 | 17 |
| Stable Disease (SD) | 18 | 8 |
| Not-assessable, too early to tell | 36 | 16 |
While these response statistics seem a little low compared to the stellar statistics we have gotten used to seeing in clinical trials such as FCR and FR for chemo naïve patients, it is important to judge them in the context of the patient cohort that is being studied here. As we pointed out in the section under “Patient Characteristics”, majority of these were fludarabine refractory and a significant percent of them had poor prognostic FISH results. So, the more relevant question to ask is this: How did our Bucket-C kids do in this clinical trial? The Roswell Park presentation at ASH 2025 had this crucial bit of information broken out.
| Prognostic Indicator | Number of Patients | Responses |
| 11q (ATM) deletion | 10 | 1 CR; 3 PR; 4 SD |
| 17p (p53) deletion | 6 | 3 PR; 1 SD |
| Fludarabine Refractory | 23 | 1 CR; 6 PR; 3 SD |
The major cause of the buzz regarding Revlimid at ASH 2025 was that it might be another drug that is effective in the treatment of poor prognostic CLL. Today’s research emphasis is leaning towards finding ways of treating patients with poor FISH cytogenetics (people with 11q (ATM) deletions, and 17p (p53) deletions). Even if you are an early stage patient and you do not have these particular nasty chromosomal abnormalities right now, chances are that at some point in time, after you have been through a couple of layers of chemotherapy, you are much more likely to have these defects in your CLL cells. In recent months we have seen papers pointing out that potent combinations such as FR (fludarabine + Rituxan) do no work very well in this group. The response statistics are lower and the remissions do not last as long for these “Bucket C” patients. Since “clonal evolution” means that most of us will eventually develop these hard-to-treat FISH defects, research done to find better drugs to treat them is welcome news to all of us.
While it is heartening to see the “CR” responses for the poor prognostic cases in this pivotal Revlimid trial, they are nevertheless not very numerous. I was hoping for much better responses for these guys, they deserve a break if anyone does. The long and sobering laundry list of adverse effects makes choice of Revlimid a little less than a slam dunk, especially in view of the slim pickings of CR responses. Frankly, it would be interesting to see how Revlimid therapy compares to combinations such as Campath + HDMP (Sledgehammer therapy reviewed for CLL Topics by Dr. Andrew Pettitt, Chairman of the UK CLL Forum) in treating poor prognostic patients. Both Campath and HDMP are known to work independently of a functioning 17p (p53) pathway. Both are known to be significantly immunosuppressive, exposing patients to opportunistic infections, hematological toxicity, etc. — but then Revlimid adverse effects are no walk in the park either. On the plus side for the “Sledgehammer”, we know a great deal more about Campath and steroids in treating CLL. Revlimid is a newcomer to this field and it will be some time before we have as much clinical information with this new drug. It is what we do not know that often comes back to bite us in uncomfortable places, and I hate surprises when it comes to cancer treatments. Don’t you?
It is a pretty well understood fact of life that most cancer drugs will eventually stop working and patients will become refractory to them. Fludarabine is a famous example. For a majority of CLL patients it works like gangbusters the first time around. But as surely as there are taxes to pay each year, patients develop resistance to the drug after a while. The words “fludarabine refractory” have an ominous sound to them, and rightly so. Patients who no longer respond to fludarabine have far fewer choices.
So, the million dollar question that has yet to be answered with regard to Revlimid is this: how soon does this drug stop working and patients become refractory to it? What bridges do we burn in the process of becoming refractory to Revlimid? For example, can patients who have flunked Revlimid (with or without Rituxan as a second drug) go on to use Campath-based therapies? As in the case of drugs such as doxorubicin, mitoxantrone, etc., do we pick up cumulative cardiac toxicity with each additional dose of Revlimid? Does this have impact on our future chances of opting for a mini-allo stem cell transplant? Patients who go into transplants with good cardiac and pulmonary function have far better chance of surviving the transplant procedure and getting long term cures. Mortality is a lot higher if you go in with a lot of baggage.
One of the more infamous side-effects of thalidomide is peripheral neuropathy (damage to nerves of the hands and feet). Thalidomide crosses the blood-brain barrier very readily and is therefore considered a psychotropic drug. It stands to reason: thalidomide was used to combat nausea in pregnancy and as a sedative. Both of these effects require modulation of brain functions. The adverse effect of peripheral nerve damage was a secondary and mostly unanticipated consequence. Revlimid is thought to be an improvement over its parent in this respect, and peripheral neuropathy is not considered a risk factor (it is, however, mentioned as a possible though low probability adverse effect).
Sitting quietly in the audience as Revlimid was discussed at the ASH 2025, I asked the question whether Revlimid too crossed the blood-brain barrier, as did its parent thalidomide, and if this meant there may be risk of so-called “chemo-brain” effect. I had reason to ask the question, since a few patients participating in the Revlimid clinical trials had written to me complaining of feeling “dopey”, forgetting stuff and generally feeling not quite on the ball after starting therapy. The meeting was chaired by Dr. Michael Keating (M. D. Anderson) and one of the other experts on the panel was Dr. Neil Kay (Mayo Clinic). Both agreed that Revlimid is likely to be equally agile in crossing the blood-brain barrier. But neither of them opined on the potential for "chemo-brain" with this new improved analog of thalidomide.
Just about every cancer patient I have ever known that has been through intensive chemotherapy treatment has felt that there was one specific adverse effect of treatment, namely the infamous “chemo brain”, that is rarely discussed or even acknowledged by oncologists. That is true for Revlimid as well. In the long list of adverse effects mentioned in the Revlimid clinical trials, I have seen no mention of monitoring for decreased cognitive function. This is nevertheless a hugely important quality of life issue for patients and I hope future research will include monitoring this aspect of Revlimid. I am only too aware that anecdotal stories from a couple of patients don’t carry much weight. But perhaps if enough of us ask the question, someone with more professional credibility and responsibility will include this question among the list of things to track in the next Revlimid clinical trial.
These are not trivial questions, and it will take some time to get credible answers to them, backed up with robust clinical data. Campath, HDMP and stem cell transplants are among the most valuable therapy options open to patients with poor prognostic indicators, and we had best be careful about not burning too many bridges on this front. “Chemo-brain” can impact a patient's quality of life significantly, if it is indeed an issue with Revlimid. These are important issues to consider as we ponder the nagging and ever-present “risks versus rewards” equation.
I have been trying to reach Dr. Asher Chanan-Khan, the lead researcher at Roswell Park, to ask him some of these questions. If I manage to reach him, and he has interesting things to say on the record, I will be sure to bring you up to speed as well.
During my long telephone conference call with Celgene Corporation folks, I became aware that Revlimid is available to CLL patients outside of formal clinical trials. If you and your treating physician are of the opinion that Revlimid therapy is the right choice for you, it is possible to get this drug outside of clinical trials, without having to travel to the study center and having to fulfill all their inclusion criteria. Here is direct quote from the company:
REVLIMID (lenalidomide) is available commercially through our RevAssist program for Revlimid education and prescribing safety. Any physician or authorized prescriber may register in the RevAssist Program by calling customer service at 1-888-423-5436 or visit www.REVLIMID.com. Revlimid will be distributed by network contract pharmacies. Only RevAssist contract pharmacies can dispense REVLIMID.
The extra restrictions on who can dispense Revlimid are entirely understandable, in view of the “thalidomide baby” concerns from an earlier age. They want to make sure physicians are fully aware that this drug is not to be used in situations where there is even a remote chance of pregnancy, and rightly so. But before you and your physician decide to use Revlimid outside of clinical trials, there are a few things I would like you to consider.
Here is the abstract of the presentation from M. D. Anderson given at the 2025 ASH Annual Meeting.
Presented at ASH 2025 Annual Meeting, Orlando, FL. Session Type: Oral Session
Lenalidomide Induces Complete and Partial Responses in Patients with Relapsed and Treatment-Refractory Chronic Lymphocytic Leukemia (CLL).
Alessandra Ferrajoli, Susan M. O'Brien, Stefan H. Faderl, William G. Wierda, Dietrich Davis, Bang-Ning Lee, James M. Reuben, Ellen J. Schlette, Hagop M. Kantarjian, Michael J. Keating
Department of Leukemia, The University of Texas, M.D. Anderson Cancer Center, Houston, TX; Department of Hematopathology, The University of Texas, M.D. Anderson Cancer Center, Houston, TX
Lenalidomide is an immunomodulatory drug indicated as treatment for 5q-MDS and previously treated MM. Several of its properties, i.e. inhibition of TNF- and VEGF, modulation of T-cell activity and impact on the microenvironment suggest that lenalidomide may play a role in the treatment of CLL. A pilot study by Chanan-Khan et al. has shown clinical activity in this disease (Blood 106: 447a). To further investigate the activity of lenalidomide in CLL, we conducted a phase II study. Patients (pts) were eligible if they had received at least one purine analog-based regimen. All pts received lenalidomide at 10 mg daily for 28 days followed by titration upward by 5 mg increments every 28 days to a maximum dose of 25 mg daily. De-escalation to 5 mg daily was allowed. The planned 35 pts have been enrolled. Twenty-two pts have received treatment for at least 3 months and are therefore evaluable for response. The median daily dose of lenalidomide at three months was 10 mg. The median age was 64 yrs (range 49-82), the median number of prior treatments was 4 (1-15), and the median 2M was 4.1 mg/dL (1.6-10.1). Twelve pts (55%) had Rai stage III or IV disease. Eight pts (36%) were refractory to fludarabine and 7 pts (32%) to alkylating agents. Responses according to NCI-WG criteria assessed after 3 months of treatment showed that 7 pts (32%) achieved a response [1 CR (5%), 1 nodular PR (5%), 5 PR (23%)]. Nine pts (41%) attained stable disease or clinical improvement and are continuing on treatment, and 6 pts (27%) progressed, including one early death that occurred on day 22 secondary to mucormycosis. A tumor flare reaction was observed in 6 pts (27%), fatigue was reported in 59% (G3 in 5%), nausea in 45%, pruritus in 31% and diarrhea in 22% (G 3 in 5%). Myelosuppression occurred in 55% of the pts (32% G3 neutropenia and/or thrombocytopenia). Infectious complications were observed in 6 pts: 2 cases of neutropenic fever and 4 episodes of pneumonia. To decipher lenalidomides mechanism of action we measured plasma levels of tumor necrosis factor (TNF)-, soluble TNF receptor 1 (TNF-R1), interferon (IFN)-, VEGF, bFGF, interleukin (IL)-1, -2, -6, -8, -10, -12, IL-1 receptor antagonist (IL-1RA), and soluble IL-2 receptor (IL-2R) in 19 pts pre-treatment, on day 28, and after 3 months of therapy. We found a significant reduction in plasma VEGF level on day 28 in 4 pts that achieved a response [mean reduction of 55.6 ( 15.3) pg/mL, p = 0.036] and on day 90 in 4 pts with stable disease or clinical improvement [mean reduction of 50.9 ( 3.3) pg/mL, p = 0.003]. There were no changes in the levels of the other cytokines, including TNF-. Further correlative studies include measurement of marrow vascularity, number of circulating T regulatory cells and CLL apoptotic rate. In conclusion, we found that lenalidomide is active in heavily pre-treated pts with relapsed CLL. Myelosuppression has been the most significant toxicity. Results of ongoing correlative studies will be presented.
Abstract #305 appears in Blood, Volume 108, issue 11, November 16, 2025
Keywords: Imids|Chronic lymphocytic leukemia|Therapy
Abstracts of Clinical Interest
Disclosures: Lenalidomide has been approved by regulatory agencies for the treatment of 5q-myelodysplastic syndrome and multiple myeloma. The use of lenalidomide in CLL should be considered off label.; Celgene provides financial support and drug supply for this study.
___________
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2025-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
